Return to Medical Biochemistry Page
Introduction
Modern molecular medicine encompasses the utilization of many molecular biological techniques in the analysis of disease, disease genes and disease gene function. The study of disease genes and their function in an unaffected individual has been possible by the development of recombinant DNA and cloning techniques. The basis of the term recombinant DNA refers to the recombining of different segments of DNA. Cloning refers to the process of preparing multiple copies of an individual type of recombinant DNA molecule. The classical mechanisms for producing recombinant molecules involves the insertion of exogenous fragments of DNA into either bacterially derived plasmid (circular double stranded autonomously replicating DNAs found in bacteria) vectors or bacteriophage (viruses that infect bacteria) based vectors. The term vector refers to the DNA molecule used to carry or transport DNA of interest into cells.
back to the top
Common Enzymes used in Molecular Biology
| Enzyme(s) | Activity | Comments |
| Restriction endonucleases | recognize specific nucleotide sequences and cleaves the DNA within or near to the recognition sequences | see below |
| Reverse transcriptase (RT) | retrovirally encoded RNA-dependent DNA polymerase | used to convert mRNA into a complimentary DNA (cDNA) copy for the purpose of cloning cDNAs |
| RNase H | recognizes RNA-DNA duplexes and randomly cleaves the phosphodiester backbone of the RNA | used primarily to cleave the mRNA strand that is annealed to the first strand of cDNA generated by reverse transcription |
| DNA polymerase | synthesis of DNA | used during most procedures where DNA synthesis is required, also used in in vitro mutagenesis |
| Klenow DNA polymerase | proteolytic fragment of DNA polymerase that lacks the 5' ----> 3' exonuclease activity | used to incorporate radioactive nucleotides into restriction enzyme generated ends of DNA, also can be used in place of DNA polymerase |
| DNA ligase | covalently attaches a free 5' phosphate to a 3' hydroxyl | used in all procedures where to molecules of DNA need to be covalently attached |
| Alkaline phosphatase | removes phosphates from 5' ends of DNA molecules | used to allow 5' ends to be subsequently radiolabeled with the g-phosphate of ATP in the presence of polynucleotide kinase, also used to prevent self-ligation of restriction enzyme digested plasmids and lambda vectors |
| Polynucleotide kinase | introduces g-phosphate of ATP to 5' ends of DNA | see above for alkaline phosphatase |
| DNase I | randomly hydrolyzes the phosphodiester bonds of double-stranded DNA | is used in the identification of regions of DNA that are bound by protein and thereby protected from DNase I digestion, also used to identify transcriptionally active regions of chromatin since they are more susceptible to DNase I digestion |
| S1 Nuclease | exonuclease that recognizes single-stranded regions of DNA | used to remove regions of single strandedness in DNA or RNA-DNA duplexes |
| Exonuclease III | exonuclease that removes nucleotides from the 3' end of DNAs | used to generate deletions in DNA for sequencing, or to map functional domains of DNA duplexes |
| Terminal transferase | DNA polymerase that requires only a 3'-OH, lengthens 3' ends with any dNTP | used to introduce homopolymeric (same dNTP) tails onto the 3' ends of DNA duplexes, also used to introduce radiolabeled nucleotides on the 3' ends of DNA |
| T3, T7, and SP6 RNA polymerases | bacterial virus encoded RNA polymerase, recognize specific nucleotide sequences for initiation of transcription | used to synthesize RNA in vitro |
| Taq and Vent DNA polymerase | thermostable DNA polymerases | used in PCR |
| Taq and Vent DNA ligases | thermostable DNA ligases | used in LCR |
back to the top
Restriction Endonucleases
Restriction endonuclease are enzymes that will recognize, bind to and hydrolyze specific nucleic acid sequences in double-stranded DNA. The term restriction endonuclease was given to this class of bacterially derived enzymes since they were identified as being involved in restricting the growth of certain bacteriophages. Bacteria are capable of modifying specific sequences within their genomes by methylation which prevents their own DNA from being recognized by the restriction enzymes encoded by their genomes. This process is termed modification and restriction. Infecting bacteriophage DNA is not modified and, hence, will be digested by the restriction endonucleases present in the bacterium.
The key to the in vitro utilization of restriction endonucleases is their strict nucleotide sequence specificity. The different enzymes are identified by being given a name indicating the bacteria from which they were isolated, e.g. the enzymes EcoRI which recognizes the sequences, 5'-GAATTC-3', was isolated from Escherichia coli. One unique feature of restriction enzymes is that the nucleotide sequences they recognize are palindromic, i.e. they are the same sequences in the 5' ---> 3' direction of both strands. Some restriction endonucleases make staggered symmetrical cuts away from the center of their recognition site within the DNA duplex, some make symmetrical cuts in the middle of their recognition site while still others cleave the DNA at a distance from the recognition sequence. Enzymes that make staggered cuts leave the resultant DNA with cohesive or sticky ends. Enzymes that cleave the DNA at the center of the recognition sequence leave blunt-ended fragments of DNA.
Any two pieces of DNA containing the same sequences within their sticky ends can anneal together and be covalently ligated together in the presence of DNA ligase. Any two blunt-ended fragments of DNA can be ligated together irrespective of the sequences at the ends of the duplexes.
back to the top
DNA Sequencing
Sequencing of DNA can be accomplished by either chemical or enzymatic means. The original technique for sequencing, Maxam and Gilbert sequencing, relies on the nucleotide-specific chemical cleavage of DNA and is not routinely used any more. The enzymatic technique, Sanger sequencing, involves the use of dideoxynucleotides (2',3'-dideoxy) that terminate DNA synthesis and is, therefore, also called dideoxy chain termination sequencing.
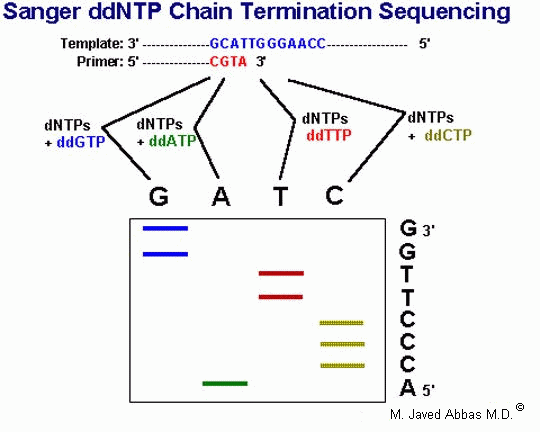 |
| The Sanger DNA sequencing protocol utilizes dideoxynucleotides (ddNTPs) to terminate chain elongation during the in vitro synthesis of DNA from a cloned template. Synthesis is initiated using a specific oligonucleotide primer. During the synthesis reaction a radioactive nucleotide (usually dATP) is incorporated into the elongating strands. Four separate reactions are carried out simultaneously, each of which contains all 4 dNTPs and a single ddNTP. The higher the concentration of ddNTP the more frequently chain elongation will terminate. Therefore, one can regulate the extent of sequence information obtainable by varying the dNTP/ddNTP ratio. Following the extension reactions the products are resolved by electrophoresis in a denaturing (urea) polyacrylamide gel. The results are obtained when the gel is dried and exposed to x-ray film. Bands near the bottom of the gel represent short reaction products (ie closest to the 3'-end of the primer) and those near the top the longest products. |
back to the top
Background to Cloning
Any fragment of DNA can be cloned once it is introduced into a suitable vector for transforming a bacterial host. Cloning refers to the production of large quantities of identical DNA molecules and usually involves the use of a bacterial cell as a host for the DNA, although cloning can be done in eukaryotic cells as well. cDNA cloning refers to the production of a library of cloned DNAs that represent all mRNAs present in a particular cell or tissue. Genomic cloning refers to the production of a library of cloned DNAs representing the entire genome of a particular organism. From either of these types of libraries one can isolate (by a variety of screening protocols) a single cDNA or gene clone.
In order to clone either cDNAs or copies of genes a vector is required to carry the cloned DNA. Vectors used in molecular biology are of two basic classes. One class of vectors is derived from bacterial plasmids. Plasmids are circular DNAs found in bacteria that replicate autonomously from the host genome. These DNAs were first identified because they harbored genes that conferred antibiotic resistance to the bacteria. The antibiotic resistance genes found on the original plasmids are used in modern in vitro engineered plasmids to allow selection of bacteria that have taken up the plasmids containing the DNAs of interest. Plasmids are limited in that in general fragments of DNA less than 10,000 base pairs (bp) can be cloned. In practice fragments of around 5,000 bp are the limit.
The second class of vectors are derived from the bacteriophage (bacterial virus) lambda. This virus is capable of both lysogeny (integration into the host genome) and lysis (infection followed by lysis of the infected host). The genes required for lysogeny have been removed from the lambda based vectors in order to allow only the lytic life cycle to take place. The advantage to lambda-based vectors is that they can carry fragments of DNA up to 25,000 bp. In the analysis of the human genome even lambda-based vectors are limiting and a yeast artificial chromosome (YAC) vector system has been developed for the cloning of DNA fragments up to 500,000 bp (see below).
back to the top
cDNA Cloning
cDNAs are made from the mRNAs of a cell by any number of related techniques. Each technique consists of first reverse transcription of the mRNA followed by synthesis of the second strand of DNA and insertion of the double-stranded cDNA into either a plasmid or lambda vector for cloning. This process creates a library of cloned cDNA representing each mRNA species. Screening of the library for a particular cDNA clone is accomplished using nucleic acid or protein-based (proteins or antibodies) probes. cDNA libraries can also be screened by biological assay of the products produced by the cloned cDNAs. Screening with proteins, antibodies or by biological assay are mechanisms for analysis of the expression of proteins from cloned cDNAs and is given the term expression cloning. Nucleic acids probes can be generated from DNA (including synthetic oligonucleotides, oligos) or RNA. Nucleic acid probes can be radioactively labeled or labeled with modified nucleotides that are recognizable by specific antibodies and detected by colorimetric or chemiluminescent assays.
A typical cDNA cloning protocol is diagrammed below.
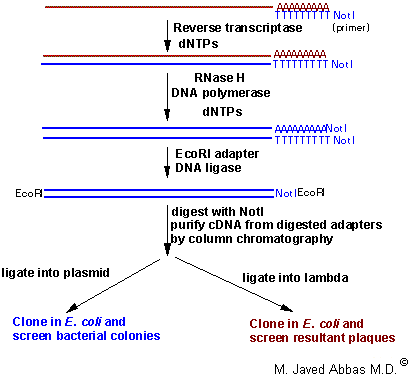 |
| Typical process for production and cloning of cDNA. This example shows the use of a specific primer-adapter containing the sequences for the restriction enzyme NotI in addition to the poly(T) for annealing to the poly(A) tail of the RNA. It is possible to use only poly(T), or poly(T) with other restriction sites or random primers (a mixture of oligos that contain random sequences) to initiate the first strand cDNA reaction. In some cases poly(T) priming does not allow for extension of the cDNA to the 5'-end of the RNA, the use of random primers can overcome this problem since they will prime first strand synthesis all along the mRNA. This technique shows the ligation of EcoRI adapters followed by EcoRI and NotI digestion. This process allows the cDNAs to all be cloned in one direction, termed directional cloning. |
back to the top
Genomic Cloning
The majority of genomic cloning utilizes lambda-based vector systems. These vector systems are capable of carrying 15-25,000 bp of DNA. Cloning slightly larger fragments of genomic DNA can be accomplished using a chimeric plasmid-lambda vector system termed a cosmid. Cosmid vectors contain only the cos (cohesive) ends of the lambda genome (required for packaging the DNA into infectious virus particles) along with a plasmid antibiotic resistance gene and origin of DNA replication. Since approximately 30,000 bp of lambda DNA have been removed from cosmid vectors, larger genomic DNA fragments can be cloned. Still larger genomic DNA fragments can be cloned into YAC vectors (see below).
Genomic DNA can be isolated from any cell or tissue for cloning. The genomic DNA is first digested with restriction enzymes to generate fragments in the size range that are optimal for the vector being utilized for cloning. Given that some genes encompass many more base pairs than can be inserted into conventional lambda or cosmid vectors, the clones that are present in a genomic library must be overlapping. In order to generate overlapping clones, the DNA is only partially digested with restriction enzymes. This means that not every restriction site, present in all the copies of a given gene in the preparation of DNA, is cleaved. The partially digested DNA is then size-selected by a variety of techniques (e.g. gel electrophoresis or gradient centrifugation) prior to cloning. Screening of genomic libraries is accomplished primarily with nucleic acid-based probes. However, they can be screened with proteins that are known to bind specific sequences of DNA (e.g. transcription factors).
A typical genomic DNA cloning protocol is diagrammed below.
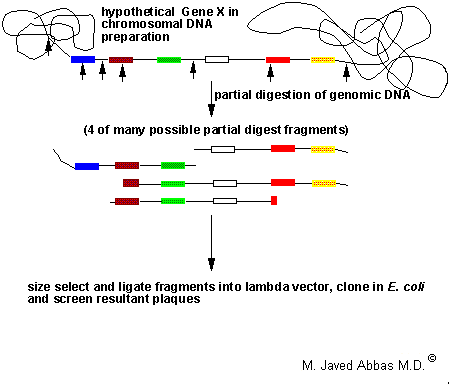 |
| Diagrammatic representation of a hypothetical gene present in a preparation of genomic DNA. The boxes indicate exons and the lines separating the boxes represent introns. The bold arrows indicate the positions of restriction enzyme sites, e.g. Sau3AI. Following partial enzyme digestion a wide range of different fragments of the gene will be generated, 4 possible fragments are indicated. Fragments in the size range of 15 - 25 kilobase pairs (kbp) are purified by gel electrophoresis or gradient centrifugation and ligated into a lambda vector. The DNA is packaged into phage particles in vitro and used to infect E. coli. |
back to the top
Cloning Genomic DNA in YAC Vectors
YAC vectors allow the cloning, within yeast cells, fragments of genomic DNA that approach 500,000 bp. These vectors contain several elements of typical yeast chromosomes, hence the term YAC. The YAC vectors contain a yeast centromere (CEN), yeast telomeres (TEL, telomeres are the specific sequences that are present at the ends of chromosomes and are necessary for replication) and a yeast autonomously replicating sequence (ARS). Yeast ARSes are essentially origins of replication that function in yeast cells autonomously from the replication of yeast chromosomal replication origins. YAC vectors also contain genes, (e.g. URA3, a gene involved in uracil synthesis) that allow selection of yeast cells that have taken up the vector. In order to propagate the vector in bacterial cells, prior to insertion of genomic DNA, YAC vectors contain a bacterial replication origin and a bacterial selectable marker such as the gene fro ampicillin resistance.
In the cloning of genomic DNA in a typical YAC vector, the genomic DNA is partially digested with EcoRI and fragments in the range of 400 - 500 kilobase pairs (kbp) are purified by pulsed field gel electrophoresis, PFGE. The YAC vector is digested with EcoRI and BamHI which places the telomere sequences at the ends of the linearized vector. The small BamHI fragment is separated from the rest of the YAC vector by standard gel electrophoresis. The genomic DNA is then ligated into the vector and then used to transform yeast cells.
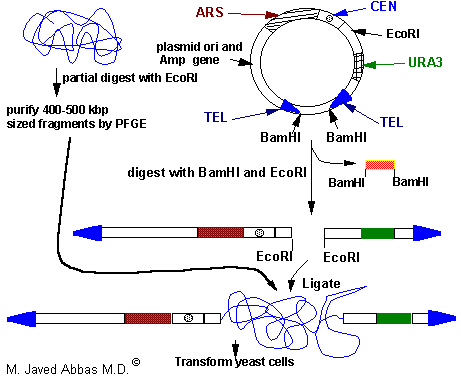 |
| Diagrammatic representation of a typical YAC vector used to clone genomic DNA. The vector contains yeast telomeres (TEL), a centromere (CEN), a selectable marker (URA3), and autonomously replicating sequences (ARS) as well as bacterial plasmid sequences for antibiotic selection and replication in E. coli.
|
back to the top
Analysis of Cloned Products
The analysis of cloned cDNAs and genes involves a number of techniques. The initial characterization usually involves mapping of the number and location of different restriction enzyme sites. This information is useful for DNA sequencing since it provides a means to digest the clone into specific fragments for sub-cloning, a process involving the cloning of fragments of a particular cloned DNA. Once the DNA is fully characterized cDNA clones can be used to produce RNA in vitro and the RNA translated in vitro to characterize the protein. Clones of cDNAs also can be used as probes to analyze the structure of a gene by Southern blotting or to analyze the size of the RNA and pattern of its expression by Northern blotting. Northern blotting is also a useful tool in the analysis of the exon-intron organization of gene clones since only fragments of a gene that contain exons will hybridize to the RNA on the blot.
Southern Blotting
Southern blotting is the analysis of DNA structure following its attachment to a solid support. The DNA to be analyzed is first digested with a given restriction enzyme then the resultant DNA fragments are separated in an agarose gel. The gel is treated with NaOH to denature the DNA, then the NaOH is neutralized. The DNA is transferred from the gel to nitrocellulose or nylon filter paper by either capillary diffusion or under electric current. The DNA is fixed to the filter by baking or ultraviolet light treatment. The filter can then be probed for the presence of a given fragment of DNA by various radioactive or non-radioactive means.
Northern Blotting
Northern blotting involves the analysis of RNA following its attachment to a solid support. The RNA is sized by gel electrophoresis then transferred to nitrocellulose or nylon filter paper as for Southern blotting. Probing the filter for a particular RNA is done similarly to probing of Southern blots.
Western Blotting
Western blotting involves the analysis of proteins following attachment to a solid support. The proteins are separated by size SDS-PAGE and electrophoretically transferred to nitrocellulose or nylon filters. The filter is then probed with antibodies raised against a particular protein.
back to the top
Restriction Fragment Length Polymorphism (RFLP) Analysis
The genetic variability at a particular locus (gene) due to even minor base changes can alter the pattern of restriction enzyme digestion fragments that can be generated. Pathogenic alterations to the genotypic can be due to deletions or insertions within the gene being analyzed or even single nucleotide substitutions that can create or delete a restriction enzyme recognition site. RFLP analysis takes advantage of this and utilizes Southern blotting of restriction enzyme digested genomic DNA to detect familial patterns of the fragments of a given gene, detectable by screening the Southern blot with a probe corresponding to the gene of interest. A classic example of a disease detectable by RFLP is sickle cell anemia.
Sickle cell anemia results (at the level of the gene) from a single nucleotide change (A --> T) at codon 6 within the b-globin gene. This alteration leads to a glu ----> val amino acid substitution, while at the same time abolishing a MstII restriction site. As a result a b-globin gene probe can be used to detect the different MstII restriction fragments. It should be recalled that there are two copies of each gene in all human cells, therefore, RFLP analysis detects both copies: the affected alelle and the unaffected allele.
Size variability in detectable fragments within a family pedigree indicate differences in the pattern of restriction sites within and around the gene being analyzed. RFLP patterns are inherited and segregate in Mendelian fashion thus, allowing their use in genotyping such as in cases of paternity dispute or in criminal investigations.
Another form of DNA polymorphism detectable by classical RFLP mapping results from the inherited variations in the number of tandemly repeated DNA sequence elements that are from 2 to 60 bp in length. The number of repeats is also variable from 2 to 40 copies. These elements are termed variable number tandem repeats (VNTRs). When restriction enzyme digestion cuts DNA flanking the VNTRs, the lengths of the resultant fragments will be variable depending upon the number of repeats at a given locus. Many different VNTR loci have been identified and are extremely useful for DNA fingerprint analysis such as in forensic and paternity identity cases.
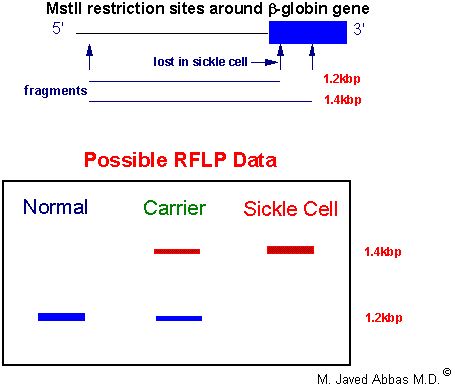 |
| Diagrammatic respresentation of an RFLP analysis for the presence of the sickle-cell locus. Genomic DNA is isolated and digested with the restriction enzyme MstII. One MstII site is lost at the sickle-cell locus. The DNA is then Southern blotted and analyzed with a b-globin-specific probe corresponding to sequences at the 5'-end of the gene. Individuals homozygous for the normal globin genes will exhibit a single hybridization band since both maternal and paternal genes are unaffected. Heterozygotes will exhibit the normal band and the larger sickle-cell gene band. Homozygous sickle-cell individuals will exhibit a single larger hybridization band.
|
back to the top
The Polymerase Chain Reaction (PCR)
The PCR is a powerful technique used to amplify DNA millions of fold, by repeated replication of a template, in a short period of time. The process utilizes sets of specific in vitro synthesized oligonucleotides to prime DNA synthesis. The design of the primers is dependent upon the sequences of the DNA that is desired to be analyzed. The technique is carried out through many cycles (usually 20 - 50) of melting the template at high temperature, allowing the primers to anneal to complimentary sequences within the template and then replicating the template with DNA polymerase. The process has been automated with the use of thermostable DNA polymerases isolated from bacteria that grow in thermal vents in the ocean or hot springs. During the first round of replication a single copy of DNA is converted to two copies and so on resulting in an exponential increase in the number of copies of the sequences targeted by the primers. After just 20 cycles a single copy of DNA is amplified over 2,000,000 fold.
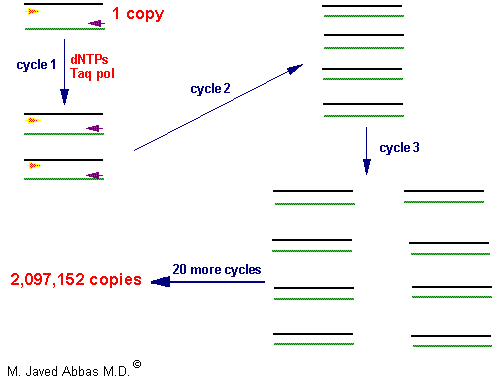 |
| The polymerase chain reaction can be used to amplify both double and single stranded (eg the products of a reverse transcription reaction, RT-PCR) DNA. The template is mixed with specific or degenerate primers, dNTPs, polymerase buffer including MgCl2 and thermostable DNA polymerase. The template is denatured at high temperature (eg 95oC) and then cooled to a temperature that will allow optimal primer binding. The reaction temperature is then raised to that optimal for the DNA polymerase (eg 72oC) whereby the primers are extended along the template. This series of steps is carried out 20 - 30 times leading to exponential amplification of the target template. The amplification is so great that the reaction products can be visualized following gel electrophoresis. |
The products of PCR reactions are analyzed by separation in agarose gels followed by ethidium bromide staining and visualization with uv transillumination. Alternatively, radioactive dNTPs can be added to the PCR in order to incorporate label into the products. In this case the products of the PCR are visualized by exposure of the gel to x-ray film. The added advantage of radiolabeling PCR products is that the levels of individual amplification products can be quantitated.
PCR can be used in the analysis of disease genes by being able to amplify detectable amounts of specific fragments of DNA. The amplified fragments from disease genes may be larger, due to insertions, or smaller, due to deletions. The dramatic amplification of DNA by PCR allows the analysis of disease genes in extremely small samples of DNA. For example, only a small number of fetal cells need be extracted from amniotic fluid in order to analyze for the presence of specific disease genes. Additionally, single point mutations can be detected by modified PCR techniques such as the ligase chain reaction (LCR) and PCR-single-strand conformational polymorphisms (PCR-SSCP) analysis. The PCR technique also can be used to identify the level of expression of genes in extremely small samples of material, e.g. tissues or cells from the body. This technique is termed reverse transcription-PCR (RT-PCR).
Examples of Inherited Disorders Detectable by PCR
Reverse Transcription-PCR (RT-PCR)
RT-PCR is a rapid and quantitative procedure for the analysis of the level of expression of genes. This technique utilizes the ability of reverse transcriptase (RT) to convert RNA into single-stranded cDNA and couples it with the PCR-mediated amplification of specific types of cDNAs present in the RT reaction. The cDNAs that are produced during the RT reaction represent a window into the pattern of genes that are being expressed at the time the RNA was extracted.
Total cellular RNA can be extracted from tissues or cells by any of several techniques and used as a template for RT. In most cases the RNA is primed using random primers. A small aliquot of the RT reaction is then added to a PCR reaction containing primers specific to the sequences one wishes to amplify. The products of the RT-PCR can be then be visualized as described above for standard PCR.
PCR-Single-Strand Conformation Polymorphism (PCR-SSCP)
Many inherited disorders are due to single nucleotide changes within critical regions of the affected gene (eg sickle cell anemia). The PCR-SSCP technique can detect single mutations in genes due to the altered conformation mobility of the single strands of DNA (within an electrophoresis gel) harboring the mutation relative to the wild-type strands that do not. Specific PCR primers are made that span the sequences of a given disease gene where a mutation is known to exist and the region amplified by PCR. The same region of the wild-type gene is PCR amplified. The two strands of wild-type PCR product will migrate differently than the two strands of mutant PCR product. Even single point mutations lead to the strands of amplified DNA existing in different conformations which alter their mobility when subjected to electrophoresis in non-denaturing gels.
In order to accurately visualize the PCR products following gel electrophoresis either the primers are radioactively labeled or radioactive nucleotides are incorporated into the PCR products. The PCR products are separated in a polyacrylamide gel and visualized by exposure of the gel to x-ray film. Individuals that are homozygous wild-type at the locus being analyzed will exhibit two distinct bands in the gel as will those individuals that are homozygous mutant. However, due to the nucleotide change the mutant PCR products will migrate with different mobilities in the gel. Individuals that are heterozygous will exhibit a pattern consisting of all four bands.
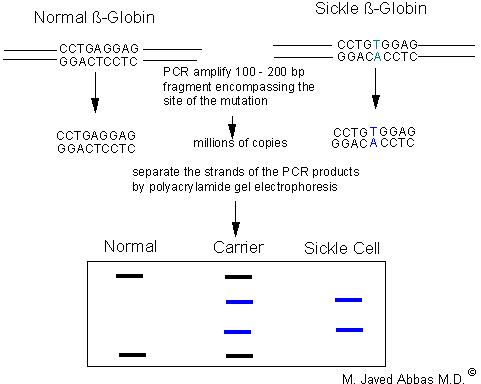 |
| PCR-SSCP analysis of normal and sickle cell b-globin genes. The A ---> T mutation is indicated in blue. The region surrounding the mutation is PCR amplified and the products separated on a non-denaturing polyacrylamide gel. The PCR products from the wild-type locus and the sickle cell locus will migrate differently due to sequence-specific conformations. Persons homozygous normal will display two bands as will homozygous sickle cell persons (although of different sizes than normal). Persons heterozygous at the sickle cell locu will display four bands.
|
back to the top
The Ligase Chain Reaction (LCR)
The LCR is another technique that allows detection of single point mutations in disease genes. The technique utilizes a thermostable DNA ligase to ligate together perfectly adjacent oligos. Two sets of oligos are designed to anneal to one strand of the gene at the site of the mutation, a second set of two oligos anneals to the other strand. The oligos are designed such that they will only completely anneal to the wild-type sequences. In the example shown below for the sickle-cell mutation, the 3' nucleotide of one oligo in each pair is mismatched. This mismatch prevent the annealing of the oligos directly adjacent to each other. Therefore, DNA ligase will not ligate the two oligos of each pair together. With the wild-type sequence the oligo pairs that are ligated together become targets for annealing the oligos and, therefore, result in an exponential amplification of the wild-type target. Given that prior sequence knowledge is required in order to detect point mutations in disease genes, the LCR technique is utilized for the diagnosis of the presence of a mutant allele in high risk patients.
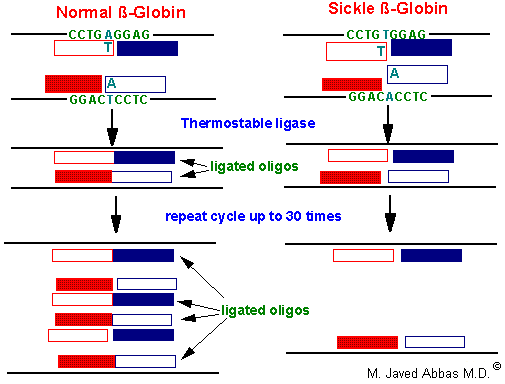 |
| The LCR technique used to analyze the sickle-cell locus. |
back to the top
Transgenesis
Transgenesis refers to the process of introducing exogenous genes into the germ line of an organism. The first successful transgenesis experiments were carried out in mice. One relatively well known experiment involved the introduction of the rat growth hormone gene into the germ line of mice. These transgenic mice grew to twice their normal size.
To create a transgenic animal the gene of interest must be passed from generation to generation, i.e. it must be inherited in the germ line. To accomplish this with mice or livestock animals, vectors containing the gene of interest with appropriate regulatory elements (e.g. the b-lactoglobulin promoter if expression of the transgene in the milk is desired) are injected into the nucleus of fertilized eggs. The eggs are then transplanted into the uterus of receptive females for development of the potential transgenic offspring. In order to test the resultant animal for germ line transmission of the transgene the chromosomal DNA of their offspring is tested for the presence of the transgene. If the transgene exhibits Mendelian inheritance then it is being transmitted in the germ line.
Currently the process of transgenesis is being utilized in both the plant and livestock industries. The aim of the majority of these experiments is to generate plants and animals that are more resistant to diseases and infections. However, some transgenic farm animal such as sheep and cows are being developed in order to obtain high levels of expression of therapeutically important proteins during milk synthesis. This allows large amounts of the protein of interest to be purified from the milk of the transgenic animals.
back to the top
Gene Therapy
Transgenesis with humans would allow for the elimination of disease genes in a population of offspring, however, technical as well as ethical issues likely will prevent any transgenic experiments to be carried out with human eggs. Therefore, the ability to replace known disease genes with normal copies in afflicted humans is the ultimate goal of gene therapy. Human gene therapy protocols aim to introduce correcting copies of disease genes into somatic cells of the affected individual. Expression of a correct copy of an affected gene in somatic cells prevents transmission through the germ line, thereby, avoiding many of the ethical issues of transgenesis. This is analogous to treatment of individuals by organ or tissue transplantation.
The most common techniques utilized in gene therapy studies is the introduction of the corrected gene into bone marrow cells, skin fibroblasts or hepatocytes. The vectors most commonly utilized are derived from retroviruses and utilize only the transcriptional promoter regions of these viruses (the LTRs) to drive expression of the gene of interest. The advantage of retroviral-based vector systems is that expression occurs in most cell types.
A number of human inherited disorders have been corrected in cultured cells and several diseases (e.g. malignant melanoma and severe combined immunodeficiency disease, SCID) are currently being treated by gene therapy techniques indicating that gene therapy is likely to be a powerful therapeutic technique against a host of diseases in coming years.
Human Disorders Treated in Cultured Cells by Gene Therapy
back to the top
Back to Topics<<<<
This article has been modified by Dr. M. Javed Abbas.
If you have any comments please do not hesitate to sign my Guest Book.
21:57 19/12/2002
|







