Myoglobin
Myoglobin and hemoglobin are hemeproteins whose physiological importance is
principally related to their ability to bind molecular oxygen. Myoglobin is a
monomeric heme protein found mainly in muscle tissue where it serves as an
intracellular storage site for oxygen. During periods of oxygen deprivation
oxymyoglobin releases its bound oxygen which is
then used for metabolic purposes.
The tertiary structure of myoglobin is that of a typical water soluble
globular protein. Its secondary structure is unusual in that it contains a very
high proportion (75%) of a-helical secondary structure.
A myoglobin polypeptide is comprised of 8 separate right handed a-helices, designated A through H, that are connected by
short non helical regions. Amino acid R-groups packed into the interior of the
molecule are predominantly hydrophobic in character while those exposed on the
surface of the molecule are generally hydrophilic, thus making the molecule
relatively water soluble.
Each myoglobin molecule contains one heme prosthetic group
inserted into a hydrophobic cleft in the protein. Each heme residue contains one
central coordinately bound iron atom that is normally in the Fe2+, or
ferrous, oxidation state. The oxygen carried by hemeproteins is bound directly
to the ferrous iron atom of the heme prosthetic group. Oxidation of the iron to
the Fe3+, ferric, oxidation state renders the molecule incapable of
normal oxygen binding. Hydrophobic interactions between the tetrapyrrole ring
and hydrophobic amino acid R groups on the interior of the cleft in the protein
strongly stabilize the heme protein conjugate. In addition a nitrogen atom from
a histidine R group located above the plane of the heme ring is coordinated with
the iron atom further stabilizing the interaction between the heme and the
protein. In oxymyoglobin the remaining bonding site on the iron atom (the 6th
coordinate position) is occupied by the oxygen, whose binding is stabilized by a
second histidine residue.
Carbon monoxide also binds coordinately to heme iron atoms in a manner
similar to that of oxygen, but the binding of carbon monoxide to heme is much
stronger than that of oxygen. The preferential binding of carbon monoxide to
heme iron is largely responsible for the asphyxiation that results from carbon
monoxide poisoning.
back
to the top
Hemoglobin
Hemoglobin is an [a(2):b(2)] tetrameric hemeprotein found in erythrocytes where it
is responsible for binding oxygen in the lung and transporting the bound oxygen
throughout the body where it is used in aerobic metabolic pathways. Each subunit
of a hemoglobin tetramer has a heme prosthetic group identical to that described
for myoglobin. The common peptide subunits are designated a, b, g and
d which are arranged into the most commonly occurring
functional hemoglobins.
Although the secondary and tertiary structure of various hemoglobin subunits
are similar, reflecting extensive homology in amino acid composition, the
variations in amino acid composition that do exist impart marked differences in
hemoglobin's oxygen carrying properties. In addition, the quaternary structure
of hemoglobin leads to physiologically important allosteric interactions between
the subunits, a property lacking in monomeric myoglobin which is otherwise very
similar to the a-subunit of hemoglobin.
Comparison of the oxygen binding properties of myoglobin and hemoglobin
(see this link from
Thomas J. Herbert) illustrate the allosteric properties of hemoglobin
that results from its quaternary structure and differentiate hemoglobin's oxygen
binding properties from that of myoglobin. The curve of oxygen binding to
hemoglobin is sigmoidal typical of allosteric proteins in which the substrate,
in this case oxygen, is a positive homotropic effector. When oxygen binds to the
first subunit of deoxyhemoglobin it increases the affinity of the remaining
subunits for oxygen. As additional oxygen is bound to the second and third
subunits oxygen binding is further, incrementally, strengthened, so that at the
oxygen tension in lung alveoli, hemoglobin is fully saturated with oxygen. As
oxyhemoglobin circulates to deoxygenated tissue, oxygen is incrementally
unloaded and the affinity of hemoglobin for oxygen is reduced. Thus at the
lowest oxygen tensions found in very active tissues the binding affinity of
hemoglobin for oxygen is very low allowing maximal delivery of oxygen to the
tissue. In contrast the oxygen binding curve for myoglobin is hyperbolic in
character indicating the absence of allosteric interactions in this process.
The allosteric oxygen binding properties of hemoglobin arise directly from
the interaction of oxygen with the iron atom of the heme prosthetic groups and
the resultant effects of these interactions on the quaternary structure of the
protein. When oxygen binds to an iron atom of deoxyhemoglobin it pulls the iron
atom into the plane of the heme. Since the iron is also bound to histidine F8,
this residue is also pulled toward the plane of the heme ring. The
conformational change at histidine F8 is transmitted throughout the peptide
backbone resulting in a significant change in tertiary structure of the entire
subunit. Conformational changes at the subunit surface lead to a new set of
binding interactions between adjacent subunits. The latter changes include
disruption of salt bridges and formation of new hydrogen bonds and new
hydrophobic interactions, all of which contribute to the new quaternary
structure.
The latter changes in subunit interaction are transmitted, from the surface,
to the heme binding pocket of a second deoxy subunit and result in easier access
of oxygen to the iron atom of the second heme and thus a greater affinity of the
hemoglobin molecule for a second oxygen molecule. The tertiary configuration of
low affinity, deoxygenated hemoglobin (Hb) is known as the taut (T) state.
Conversely, the quaternary structure of the fully oxygenated high affinity form
of hemoglobin (HbO2) is known as the relaxed (R) state.
If using a Javascript supporting browser one can see the
T to R transition at this site.
In addition to transporting oxygen from lungs to peripheral tissues,
hemoglobin molecules play an important role in transporting CO2 in
the opposite direction. N-terminal amino groups of the T form of hemoglobin are
available for reaction with CO2 and about 15% of the CO2
formed in tissues is carried to the lung covalently bound to the N-terminal
nitrogens as carbamate:
CO2 + Hb-NH2 <-----> H+ +
Hb-NH-COO-
In the lung the high oxygen partial pressure favors formation of the R form
resulting in reversal of the carbamate with release and exhalation of the
CO2.
During the conversion from T form to R form several R groups on the surface
of hemoglobin subunits also become available to dissociate protons:
4O2 + Hb <--------> nH+ +
Hb(O2)4
The above two equations illustrate that the conformation of hemoglobin and
its oxygen binding are sensitive to hydrogen ion concentration. These effects of
hydrogen ion concentration are responsible for the well known Bohr effect in which increases in hydrogen ion concentration
decrease the amount of oxygen bound by hemoglobin at any oxygen concentration
(partial pressure).
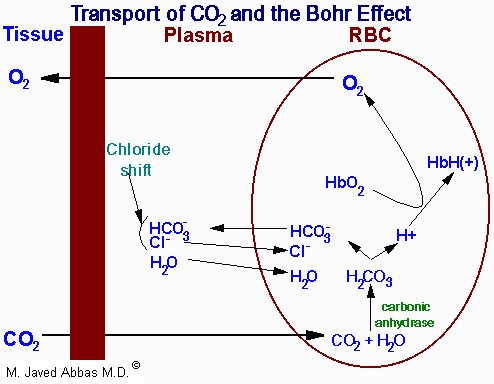 |
| Representation of the transport of
CO2 from the tissues to the blood with delivery of
O2 to the tissues. The opposite process occurs when
O2 is taken up from the alveoli of the lungs and the
CO2 is expelled. All of the processes of the transport of
CO2 and O2 are not shown such as the formation and
ionization of carbonic acid in the plasma. The latter is a major mechanism
for the transport of CO2 to the lungs, i.e. in the plasma as
HCO3-. The H+ produced in the plasma by
the ionization of carbonic acid is buffered by phosphate
(HPO42-) and by proteins. Additionally, some 15% of
the CO2 is transported from the tissues to the lungs as hemoglobin carbamate.
|
Tissue CO2 is also carried to the lungs as the dissolved gas and
as bicarbonate formed spontaneously and by the enzyme carbonic
anhydrase which converts CO2 and H2O to carbonic
acid. The carbonic acid thus formed spontaneously ionizes to proton and
bicarbonate ion:
CO2 + H2O --------> H2CO3
------> H+ + HCO3-
back
to the top
Role of 2,3-bisphosphoglycerate (2,3-BPG)
The compound 2,3-bisphosphoglycerate
(2,3-BPG), derived from the glycolytic intermediate
1,3-bisphosphoglycerate, is a potent allosteric effector on the oxygen binding
properties of hemoglobin.
The formation of 2,3-BPG is diagrammed. In the
deoxygenated T conformer, a cavity capable of binding 2,3-BPG forms in the
center of the molecule. 2,3-BPG can occupy this cavity stabilizing the T state.
Conversely, when 2,3-BPG is not available, or not bound in the central cavity,
Hb can be converted to HbO2 more readily. Thus, like increased
hydrogen ion concentration, increased 2,3-BPG concentration favors conversion of
R form Hb to T form Hb and decreases the amount of oxygen bound by Hb at any
oxygen concentration. Hemoglobin molecules differing in subunit composition are
known to have different 2,3-BPG binding properties with correspondingly
different allosteric responses to 2,3-BPG. For example, HbF (the fetal form of
hemoglobin) binds 2,3-BPG much less avidly than HbA (the adult form of
hemoglobin) with the result that HbF in fetuses of pregnant women binds oxygen
with greater affinity than the mothers HbA, thus giving the fetus preferential
access to oxygen carried by the mothers circulatory system.
back
to the top
Back to Topics<<<<
This article has been modified by Dr. M. Javed Abbas.
If you have any comments please do not hesitate to sign my Guest Book.
20:37 21/12/2002
|
