Introduction
As a class, the nucleotides may be considered one of the most important
metabolites of the cell. Nucleotides are found primarily as the monomeric units
comprising the major nucleic acids of the cell, RNA and DNA. However, they also
are required for numerous other important functions within the cell. These
functions include:
- serving as energy stores for future use in phosphate transfer
reactions. These reactions are predominantly carried out by ATP.
- forming a portion of several important coenzymes such as
NAD+, NADP+, FAD and coenzyme A.
- serving as mediators of numerous important cellular processes
such as second messengers in signal transduction
events. The predominant second messenger is cyclic-AMP (cAMP), a cyclic
derivative of AMP formed from ATP.
- controlling numerous enzymatic reactions through allosteric
effects on enzyme activity.
- serving as activated intermediates in numerous biosynthetic
reactions. These activated intermediates include S-adenosylmethionine
(S-AdoMet) involved in methyl transfer reactions as well as the many sugar
coupled nucleotides involved in glycogen
and glycoprotein
synthesis.
back to the
top
Nucleoside and Nucleotide Structure and
Nomenclature
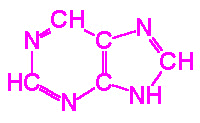
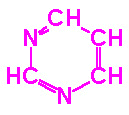
The nucleotides found in cells are derivatives of the heterocyclic highly
basic, compounds, purine and pyrimidine.
| 
|
| Purine |
Pyrimidine |
It is the chemical basicity of the nucleotides that has given them the
common term "bases" as they are associated with nucleotides present in DNA and
RNA. There are five major bases found in cells. The derivatives of purine are
called adenine and guanine, and the derivatives of pyrimidine are called
thymine, cytosine
and uracil. The common abbreviations used for
these five bases are, A, G, T, C and U.
Base
Formula |
Base
(X=H) |
Nucleoside
X=ribose or
deoxyribose |
Nucleotide
X=ribose phosphate |
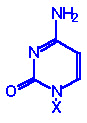 |
Cytosine, C |
Cytidine, A |
Cytidine monophosphate
CMP |
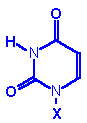 |
Uracil, U |
Uridine, U |
Uridine monophosphate
UMP |
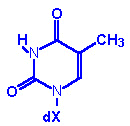 |
Thymine, T |
Thymidine, T |
Thymidine monophosphate
TMP |
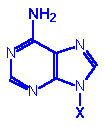 |
Adenine, A |
Adenosine, A |
Adenosine monophosphate
AMP |
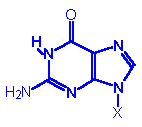 |
Guanine, G |
Guanosine, A |
Guanosine
monophosphate
GMP |
The purine and pyrimidine bases in cells are linked to carbohydrate and in
this form are termed, nucleosides. The nucleosides
are coupled to D-ribose or 2'-deoxy-D-ribose through a b-N-glycosidic bond between the anomeric carbon of the ribose
and the N9 of a purine or N1 of a pyrimidine.
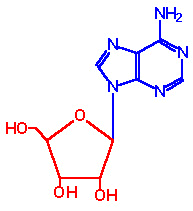
The base can exist in 2 distinct orientations about the N-glycosidic bond.
These conformations are identified as, syn
and anti. It is the anti conformation that
predominates in naturally occurring nucleotides.
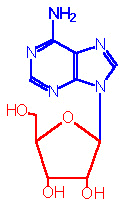
| 
|
| syn-Adenosine |
anti-Adenosine |
Nucleosides are found in the cell primarily in their phosphorylated form.
These are termed nucleotides. The most common site
of phosphorylation of nucleotides found in cells is the hydroxyl group attached
to the 5'-carbon of the ribose The carbon atoms of the ribose present in
nucleotides are designated with a prime (') mark
to distinguish them from the backbone numbering in the bases. Nucleotides can
exist in the mono-, di-, or tri-phosphorylated forms.
Nucleotides are given distinct abbreviations to allow easy identification of
their structure and state of phosphorylation. The monophosphorylated form of
adenosine (adenosine-5'-monophosphate) is written as, AMP. The di- and tri-phosphorylated forms are written as,
ADP and ATP,
respectively. The use of these abbreviations assumes that the nucleotide is in
the 5'-phosphorylated form. The di- and tri-phosphates of nucleotides are linked
by acid anhydride bonds. Acid anhydride bonds have a high DG0' for hydrolysis imparting upon them a high
potential to transfer the phosphates to other molecules. It is this property of
the nucleotides that results in their involvement in group transfer reactions in
the cell.
The nucleotides found in DNA are unique from those of RNA in that the ribose
exists in the 2'-deoxy form and the abbreviations of the nucleotides contain a
d designation. The monophosphorylated form of
adenosine found in DNA (deoxyadenosine-5'-monophosphate) is written as dAMP.
The nucleotide uridine is never found in DNA and thymine is almost
exclusively found in DNA. Thymine is found in tRNAs but not rRNAs nor mRNAs.
There are several less common bases found in DNA and RNA. The primary modified
base in DNA is 5-methylcytosine. A variety of modified bases appear in the
tRNAs. Many modified nucleotides are encountered outside of the context of DNA
and RNA that serve important biological functions.
back to the
top
Adenosine Derivatives
The most common adenosine derivative is the cyclic form, 3'-5'-cyclic adenosine monophosphate, cAMP. This compound
is a very powerful second messenger involved in
passing signal
transduction events from the cell surface to internal proteins, e.g. cAMP-dependent protein
kinase (PKA). PKA phosphorylates a number of proteins, thereby,
affecting their activity either positively or negatively. Cyclic-AMP is also
involved in the regulation of ion channels by direct interaction with the
channel proteins, e.g. in the activation of odorant receptors by odorant
molecules.
Formation of cAMP occurs in response to activation of receptor coupled
adenylate cyclase. These receptors can be of any type, e.g.
hormone receptors or odorant receptors.
S-adenosylmethionine is a form of
activated methionine which serves as a methyl donor in methylation
reactions and as a source of propylamine in the synthesis of polyamines.
back to the
top
Guanosine Derivatives
A cyclic form of GMP (cGMP) also is found in
cells involved as a second messenger molecule. In many cases its' role is to
antagonize the effects of cAMP. Formation of cGMP occurs in response to receptor
mediated signals similar to those for activation of adenylate cyclase. However,
in this case it is guanylate cyclase that is coupled to the
receptor.
The most important cGMP coupled signal transduction cascade is that photoreception.
However, in this case activation of rhodopsin (in
the rods) or other opsins (in the cones) by the
absorption of a photon of light (through 11-cis-retinal covalently
associated with rhodopsin and opsins) activates transducin which in turn
activates a cGMP specific phosphodiesterase that hydrolyzes cGMP to GMP. This
lowers the effective concentration of cGMP bound to gated ion channels resulting
in their closure and a concomitant hyperpolarization of the cell.
back to the
top
Synthetic Nucleotide Analogs
Many nucleotide analogues are chemically synthesized and used for their
therapeutic potential. The nucleotide analogues can be utilized to inhibit
specific enzymatic activities. A large family of analogues are used as
anti-tumor agents, for instance, because they interfere with the synthesis of
DNA and thereby preferentially kill rapidly dividing cells such as tumor cells.
Some of the nucleotide analogues commonly used in chemotherapy are
6-mercaptopurine, 5-fluorouracil, 5-iodo-2'-deoxyuridine and 6-thioguanine. Each
of these compounds disrupts the normal replication process by interfering with
the formation of correct Watson-Crick base-pairing.
Nucleotide analogs also have been targeted for use as antiviral agents.
Several analogs are used to interfere with the replication of HIV, such as
AZT (azidothymidine) and ddI
(dideoxyinosine).
Several purine analogs are used to treat gout. The most common is allopurinol, which resembles hypoxanthine. Allopurinol
inhibits the activity of xanthine oxidase, an enzyme involved in
de novo purine
biosynthesis. Additionally, several nucleotide analogues are used after
organ transplantation in order to suppress the immune system and reduce the
likelihood of transplant rejection by the host.
back to the
top
Polynucleotides
Polynucleotides are formed by the condensation of two or more nucleotides.
The condensation most commonly occurs between the alcohol of a 5'-phosphate of
one nucleotide and the 3'-hydroxyl of a second, with the elimination of
H2O, forming a phosphodiester bond. The
formation of phosphodiester bonds in DNA and RNA exhibits directionality. The
primary structure of DNA and RNA (the linear arrangement of the nucleotides)
proceeds in the 5' ----> 3' direction. The common representation of the
primary structure of DNA or RNA molecules is to write the nucleotide sequences
from left to right synonymous with the 5' -----> 3' direction as shown:
5'-pGpApTpC-3'
back to the
top
Structure of DNA
Utilizing X-ray diffraction data, obtained from crystals of DNA, James
Watson and Francis Crick proposed a model for the structure of DNA. This model
(subsequently verified by additional data) predicted that DNA would exist as a
helix of two complementary antiparallel strands, wound around each other in a
rightward direction and stabilized by H-bonding between bases in adjacent
strands. In the Watson-Crick model, the bases are
in the interior of the helix aligned at a nearly 90 degree angle relative to the
axis of the helix. Purine bases form hydrogen bonds with pyrimidines, in the
crucial phenomenon of base pairing. Experimental
determination has shown that, in any given molecule of DNA, the concentration of
adenine (A) is equal to thymine (T) and the concentration of cytidine (C) is
equal to guanine (G). This means that A will only base-pair with T, and C with
G. According to this pattern, known as Watson-Crick
base-pairing, the base-pairs composed of G and C contain three
H-bonds, whereas those of A and T contain two H-bonds. This makes G-C base-pairs
more stable than A-T base-pairs.
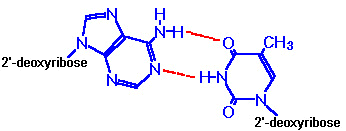
A-T Base
Pair
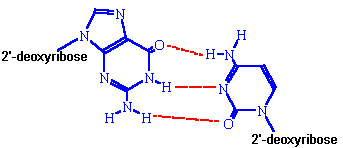
G-C Base
Pair
The antiparallel nature of the helix stems from the orientation of the
individual strands. From any fixed position in the helix, one strand is oriented
in the 5' ---> 3' direction and the other in the 3' ---> 5' direction. On
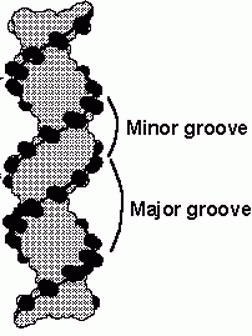
its exterior surface, the double helix of DNA contains two deep grooves between
the ribose-phosphate chains. These two grooves are of unequal size and termed
the major and minor
grooves. The difference in their size is due to the asymmetry of the
deoxyribose rings and the structurally distinct nature of the upper surface of a
base-pair relative to the bottom surface.
The double helix of DNA has been shown to exist in several different forms,
depending upon sequence content and ionic conditions of crystal preparation. The
B-form of DNA prevails under physiological
conditions of low ionic strength and a high degree of hydration. Regions of the
helix that are rich in pCpG dinucleotides can exist in a novel left-handed
helical conformation termed Z-DNA. This
conformation results from a 180 degree change in the orientation of the bases
relative to that of the more common A- and B-DNA.
 |
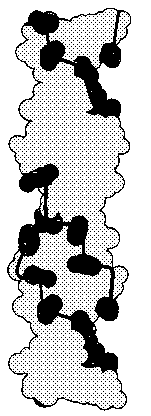 |
| Structure of B-DNA
|
Structure of
Z-DNA |
Parameters of Major DNA Helices
| Parameters |
A Form |
B Form |
Z-Form |
| Direction of helical rotation |
Right |
Right |
Left |
| Residues per turn of helix |
11 |
10 |
12 base pairs |
| Rotation of helix per residue (in
degrees) |
33 |
36 |
-30 |
| Base tilt relative to helix axis (in
degrees) |
20 |
6 |
7 |
| Major groove |
narrow and deep |
wide and deep |
Flat |
| Minor groove |
wide and shallow |
narrow and deep |
narrow and deep |
| Orientation of N-glycosidic Bond |
Anti |
Anti |
Anti for Py, Syn for Pu |
| Comments |
|
most prevalent within cells |
occurs in stretches of alternating
purine-pyrimidine base pairs |
back to the
top
Thermal Properties of DNA
As cells divide it is a necessity that the DNA be copied (replicated), in
such a way that each daughter cell acquires the same amount of genetic material.
In order for this process to proceed the two strands of the helix must first be
separated, in a process termed denaturation. This process can also be carried
out in vitro. If a solution of DNA is subjected to high temperature, the H-bonds
between bases become unstable and the strands of the helix separate in a process
of thermal denaturation.
The base composition of DNA varies widely from molecule to molecule and even
within different regions of the same molecule. Regions of the duplex that have
predominantly A-T base-pairs will be less thermally stable than those rich in
G-C base-pairs. In the process of thermal denaturation, a point is reached at
which 50% of the DNA molecule exists as single strands. This point is the
melting temperature (TM), and is
characteristic of the base composition of that DNA molecule. The TM
depends upon several factors in addition to the base composition. These include
the chemical nature of the solvent and the identities and concentrations of ions
in the solution.
When thermally melted DNA is cooled, the complementary strands will again
re-form the correct base pairs, in a process is termed annealing or hybridization.
The rate of annealing is dependent upon the nucleotide sequence of the two
strands of DNA.
back to the
top
Analysis of DNA Structure
Chromatography: Several of the chromatographic techniques
available for the characterization
of proteins can also be applied to the characterization of DNA. The most
commonly used technique is HPLC (high performance liquid chromatography).
Affinity chromatographic techniques also can be employed. One common affinity
matrix is hydroxyapatite (a form of calcium phosphate), which binds
double-stranded DNA with a higher affinity than single-stranded DNA.
back to the
top
Analysis of DNA Structure
Electrophoresis: This procedure can serve the same function
with regard to DNA molecules as it does for the analysis
of proteins. However, since DNA molecules have much higher molecular weights
than proteins, the molecular sieve used in electrophoresis of DNA must be
different as well. The material of choice is agarose, a carbohydrate polymer
purified from a salt water algae. It is a copolymer of mannose and galactose
that when melted and re-cooled forms a gel with pores sizes dependent upon the
concentration of agarose. The phosphate backbone of DNA is highly negatively
charged, therefore DNA will migrate in an electric field. The size of DNA
fragments can then be determined by comparing their migration in the gel to
known size standards. Extremely large molecules of DNA (in excess of 106 base
pairs) are effectively separated in agarose gels using pulsed-field gel electrophoresis (PFGE). This technique
employs two or more electrodes, placed orthogonally with respect to the gel,
that receive short alternating pulses of current. PFGE allows whole chromosomes
and large portions of chromosomes to be analyzed.
back to the
top
Back to Biomolecules
Back to Topics<<<<
This article has been modified by Dr. M. Javed Abbas.
If you have any comments please do not hesitate to sign my Guest Book.
20:36 21/12/2002
|












