Structure and Function of Hormones
The integration of body functions in humans and other higher organisms is carried out by the nervous system, the immune system, and the endocrine system. The endocrine system is composed of a number of tissues that secrete their products, called endocrine hormones, into the circulatory system; from there they are disseminated throughout the body, regulating the function of distant tissues and maintaining homeostasis. In a separate but related system, exocrine tissues secrete their products into ducts and then to the outside of the body or to the intestinal tract. Classically, endocrine hormones are considered to be derived from amino acids, peptides, or sterols and to act at sites distant from their tissue of origin. However, the latter definition has begun to blur as it is found that some secreted substances act at a distance (classical endocrines), close to the cells that secrete them (paracrines), or directly on the cell that secreted them (autocrines). Insulin-like growth factor-I (IGF-I), which behaves as an endocrine, paracrine, and autocrine, provides a prime example of this difficulty.
Hormones are normally present in the plasma and interstitial tissue at concentrations in the range of 10-7M to 10-10M. Because of these very low physiological concentrations, sensitive protein receptors have evolved in target tissues to sense the presence of very weak signals. In addition, systemic feedback mechanisms have evolved to regulate the production of endocrine hormones.
Once a hormone is secreted by an endocrine tissue, it generally binds to a specific plasma protein carrier, with the complex being disseminated to distant tissues. Plasma carrier proteins exist for all classes of endocrine hormones. Carrier proteins for peptide hormones prevent hormone destruction by plasma proteases. Carriers for steroid and thyroid hormones allow these very hydrophobic substances to be present in the plasma at concentrations several hundred-fold greater than their solubility in water would permit. Carriers for small, hydrophilic amino acid--derived hormones prevent their filtration through the renal glomerulus, greatly prolonging their circulating half-life.
Tissues capable of responding to endocrines have 2 properties in common: they posses a receptor having very high affinity for hormone, and the receptor is coupled to a process that regulates metabolism of the target cells. Receptors for most amino acid--derived hormones and all peptide hormones are located on the plasma membrane. Activation of these receptors by hormones (the first messenger) leads to the intracellular production of a second messenger, such as cAMP, which is responsible for initiating the intracellular biological response. Steroid and thyroid hormones are hydrophobic and diffuse from their binding proteins in the plasma, across the plasma membrane to intracellularly localized receptors. The resultant complex of steroid and receptor bind to response elements of nuclear DNA, regulating the production of mRNA for specific proteins.
back to the top
Receptors for Peptide Hormones
With the exception of the thyroid hormone receptor, the receptors for amino acid--derived and peptide hormones are located in the plasma membrane. Receptor structure is varied: some receptors consist of a single polypeptide chain with a domain on either side of the membrane, connected by a membrane-spanning domain. Some receptors are comprised of a single polypeptide chain that is passed back and forth in serpentine fashion across the membrane, giving multiple intracellular, transmembrane, and extracellular domains. Other receptors are composed of multiple polypeptides. For example, the insulin receptor is a disulfide-linked tetramer with the b subunits spanning the membrane and the a subunits located on the exterior surface.
Subsequent to hormone binding, a signal is transduced to the interior of the cell, where second messengers and phosphorylated proteins generate appropriate metabolic responses. The main second messengers are cAMP, Ca2+, inositol triphosphate (IP3) , and diacylglycerol (DAG) . Proteins are phosphorylated on serine and threonine by cAMP-dependent protein kinase (PKA) and DAG-activated protein kinase C (PKC). Additionally a series of membrane-associated and intracellular tyrosine kinases phosphorylate specific tyrosine residues on target enzymes and other regulatory proteins.
The hormone-binding signal of most, but not all, plasma membrane receptors is transduced to the interior of cells by the binding of receptor-ligand complexes to a series of membrane-localized GDP/GTP binding proteins known as G-proteins. The classic interactions between receptors, G-protein transducer, and membrane-localized adenylate cyclase are illustrated using the pancreatic hormone glucagon as an example. When G-proteins bind to receptors, GTP exchanges with GDP bound to the a subunit of the G-protein. The Ga-GTP complex binds adenylate cyclase, activating the enzyme. The activation of adenylate cyclase leads to cAMP production in the cytosol and to the activation of PKA, followed by regulatory phosphorylation of numerous enzymes. Stimulatory G-proteins are designated Gs, inhibitory G-proteins are designated Gi.
A second class of peptide hormones induces the transduction of 2 second messengers, DAG and IP3. Hormone binding is followed by interaction with a stimulatory G-protein which is followed in turn by G-protein activation of membrane-localized phospholipase C-g, (PLC-g). PLC-g hydrolyzes phosphatidylinositol bisphosphate to produce 2 messengers: IP3, which is soluble in the cytosol, and DAG, which remains in the membrane phase. Cytosolic IP3 binds to sites on the endoplasmic reticulum, opening Ca2+ channels and allowing stored Ca2+ to flood the cytosol. There it activates numerous enzymes, many by activating their calmodulin or calmodulin-like subunits. DAG has 2 roles: it binds and activates PKC, and it opens Ca2+ channels in the plasma membrane, reinforcing the effect of IP3. Like PKA, PKC phosphorylates serine and threonine residues of many proteins, thus modulating their catalytic activity.
Only 1 receptor class, that for the natriuretic factors (e.g. atrial natriuretic factor, ANF), has been shown to be coupled to the production of intracellular cGMP. ANF, a peptide secreted by cardiac atrial tissue, is much like other peptide hormones in that it is secreted into the circulatory system and has effects on distant tissue. The principal site of ANF action is the kidney glomerulus, where it modulates the rate of filtration, increasing Na+ excretion in the urine. The receptors for the natriuretic factors are integral plasma membrane proteins, whose intracellular domains catalyze the formation of cGMP following natriuretic factor-binding. Intracellular cGMP activates a protein kinase G (PKG), which phosphorylates and modulates enzyme activity, leading to the biological effects of the natriuretic factors.
back to the top
Basics of Peptide Hormones
Many amino acid and peptide hormones are elaborated by neural tissue, with ultimate impact on the entire system. When their composition was still unknown, hypothalamic secretory products were known as releasing factors, since their effect was to release endocrine hormones from the pituitary. More recently the releasing factors have been renamed releasing hormones. Currently, both names are in common use.
Releasing hormones are synthesized in neural cell bodies of the hypothalamus and secreted at the axon terminals into the portal hypophyseal circulation, which directly bathes the anterior pituitary. These peptides initiate a cascade of biochemical reactions that culminate in hormone-regulated, whole-body biological end points. Cells of the anterior pituitary, with specific receptors for individual releasing hormones, generally respond through a Ca2+, IP3, PKC-linked pathway that stimulates exocytosis of preexisting vesicles containing the various anterior pituitary hormones. The pituitary hormones are carried via the systemic circulation to target tissues throughout the body. At the target tissues they generate unique biological activities.
The secretion of hypothalamic, pituitary, and target tissue hormones is under tight regulatory control by a series of feedback and feed- forward loops. This complexity can be demonstrated using the growth hormone (GH) regulatory system as an example. The stimulatory substance growth hormone releasing hormone (GRH), and the inhibitory substance somatostatin (SS), both products of the hypothalamus, control pituitary GH secretion. Under the influence of GRH, growth hormone is released into the systemic circulation, causing the target tissue to secrete IGF-1. (Growth hormone also has other more direct metabolic effects; it is both hyperglycemic and lipolytic). The principal source of systemic IGF-1 is the liver, although most other tissues secrete and contribute to systemic IGF-1. Liver IGF-1 is considered to be the principal regulator of tissue growth. In particular, the IGF-1 secreted by the liver is believed to synchronize growth throughout the body, resulting in a homeostatic balance of tissue size and mass. IGF-1 secreted by peripheral tissues is generally considered to be autocrine or paracrine in its biological action.
Systemic IGF-1 also has hypothalamic and pituitary regulatory targets. The negative feedback loops cause down-regulation of GH secretion directly at the pituitary. The longer positive feedback loop, involving IGF-1 regulation at the hypothalamus, stimulates the secretion of somatostatin (SS, also called growth hormone-inhibiting hormone, GIH), which in turn inhibits the secretion of growth hormone by the pituitary. The latter is a relatively unusual negative feed-forward regulatory process. In addition, a shorter negative feedback loop is shown that involves direct IGF-1 action on the pituitary, leading to down-regulation of GH secretion. Similar feedback loops exist for all the major endocrine hormones, and many subtle nuances modulate each regulatory loop.
back to the top
Growth Hormone
Human placental lactogen (hPL), GH, and prolactin (Prl) comprise the growth hormone family. All have about 200 amino acids, 2 disulfide bonds, and no glycosylation. Although each has special receptors and unique characteristics to their activity, they all possess growth-promoting and lactogenic activity. Mature GH (22,000 daltons) is synthesized in acidophilic pituitary somatotropes as a single polypeptide chain. Because of alternate RNA splicing, a small amount of a somewhat smaller molecular form is also secreted.
While details of the method of signal transduction by the members of the GH family of tropic hormones remain unclear, PKC activity has been demonstrated to correlate directly with the biological effects of Prl and GH. This appears to indicate that the PKC signal transduction pathway is operative in transducing signals for the GH family of hormones.
The role of growth hormone in regulating IGF-1 production was noted above. Humans respond to natural or recombinant human or primate growth hormone with appropriate secretion of IGF-1, but growth hormone of other species has no normal biological effect in man. The latter is puzzling because interspecies GH homologies are quite high in many cases, and most other species respond well to human growth hormone. In humans, growth hormone promotes gluconeogenesis and is consequently hyperglycemic. It promotes amino acid uptake by cells, with the result that GH therapy puts an organism into positive nitrogen balance, similar to that seen in growing children. Finally, growth hormone is lipolytic, inducing the breakdown of tissue lipids and thus providing energy supplies that are used to support the stimulated protein synthesis induced by increased amino acid uptake.
There are a number of genetic deficiencies associated with GH. GH-deficient dwarfs lack the ability to synthesize or secrete GH, and these short-statured individuals respond well to GH therapy. Pygmies lack the IGF-1 response to GH but not its metabolic effects; thus in pygmies the deficiency is post-receptor in nature. Finally, Laron dwarfs have normal or excess plasma GH, but lack liver GH receptors and have low levels of circulating IGF-1. The defect in these individuals is clearly related to an inability to respond to GH by the production of IGF-1. The production of excessive amounts of GH before epiphyseal closure of the long bones leads to gigantism, and when GH becomes excessive after epiphyseal closure, acral bone growth leads to the characteristic features of acromegaly.
back to the top
Prolactin (PRL)
Prolactin is produced by acidophilic pituitary lactotropes. Prolactin is the lone tropic hormone of the pituitary that is routinely under negative control by prolactin inhibiting hormone (PIH), which is now known to be dopamine. Decreased hypophyseal dopamine production, or damage to the hypophyseal stalk, leads to rapid up-regulation of PRL secretion. A number of other hypothalamic releasing hormones induce increased prolactin secretion; as a result, it is unclear whether a specific PRH exists for up-regulating PRL secretion. PRL initiates and maintains lactation in mammals, but normally only in mammary tissue that has been primed with estrogenic sex hormones.
back to the top
Human Placental Lactogen (hPL)
Human placental lactogen is produced by the placenta late in gestation. At its height it is secreted at a rate of about 1 g/day, the highest secretory rate of any known human hormone. However, little hPL reaches the fetal circulation, and hPL has only about 1% the activity of PRL or GH in producing biological effects, leading some to question its functional importance in humans.
back to the top
The Gonadotropins
The glycoprotein hormones are the most chemically complex family of the peptide hormones. All members of the family are highly glycosylated. Each of the glycoprotein hormones is an (a:b) heterodimer, with the a subunit being identical in all members of the family. The biological activity of the hormone is determined by the b-subunit, which is not active in the absence of the a subunit. The molecular weight of the gonadotropins FSH, LH, and CG is about 25,000, whereas that of the thyroid tropic hormone TSH is about 30,000. All members of the glycoprotein family transduce their intracellular effects via the receptor, G-protein, adenylate cyclase, second-messenger system.
The gonadotropins (LH, FSH and CG) bind to cells in the ovaries and testes, stimulating the production of the steroid sex hormones estrogen, testosterone (T), and dihydrotestosterone (DHT). In males, luteinizing hormone (LH) binds to Leydig cells of the testes to induce the secretion of T, while follicle stimulating hormone (FSH) binds to Sertoli cells and induces the secretion of T and DHT. In females, LH induces thecal cells to secrete estradiol, and FSH stimulates estrogen synthesis by granulosa cells. The synthesis of the sex hormones is reviewed is covered in the steroid hormone page.
Human chorionic gonadotropin (hCG) is a placental hormone. The production of hCG increases markedly after implantation; its appearance in the plasma and urine is one of the earliest signals of pregnancy and the basis of many pregnancy tests.
back to the top
Thyroid Stimulating Hormone (TSH)
Secretion of TSH, the final member of the glycoprotein hormone family, is stimulated by TRH from the hypothalamus. While cAMP causes increased secretion of TSH by thyrotropes, it is not yet certain that cAMP is the physiological signal that regulates TSH production.
Circulating TSH binds to receptors on the basal membrane of thyroid follicles. The receptors are coupled through a G-protein to adenylate cyclase. The result is that ligand binding increases thyrocyte cAMP and PKA, leading in the short term to increased secretion of thyroxin (T4) and triiodothyronine (T3). Chronic stimulation of the receptor causes an increase in the synthesis of a major thyroid hormone precursor, thyroglobulin.
Thyroglobulin produced on rough endoplasmic reticulum has a molecular weight of 660,000. It is glycosylated and contains more than 100 tyrosine residues, which become iodinated and are used to synthesize T3 and T4. Thyroglobulin is exoctosed through the apical membrane into the closed lumen of thyroid follicles, where it accumulates as the major protein of the thyroid and where maturation takes place. Briefly, a Na+/K+-ATPase-driven pump concentrates iodide (I-) in thyroid cells, and the iodide is transported to the follicle lumen. There it is oxidized to I+ by a thyroperoxidase found only in thyroid tissue. The addition of I+ to tyrosine residues of thyroglobulin is catalyzed by the same enzyme, leading to the production of thyroglobulin containing monoiodotyrosyl (MIT) and diiodotyrosyl (DIT) residues. The thyronines, T3 and T4, are formed by combining MIT and DIT residues on thyroglobulin.
Mature, iodinated thyroglobulin is taken up in vesicles by thyrocytes and fuses with lysosomes. Lysosomal proteases degrade thyroglobulin releasing amino acids and T3 and T4, which are secreted into the circulation. These compounds are very hydrophobic and require a carrier protein for delivery to target tissues. In the plasma, T3 and T4 are bound to a carrier glycoprotein known as thyroxin-binding globulin and are disseminated throughout the body in this form.
Thyroid hormones act by binding to cytosolic receptors very similar to steroid hormone receptors, and for this reason T3 and T4 are often classified along with the hydrophobic steroid hormones. The principal role of thyroid hormones is also like that of steroid hormones. In adults, the ligand receptor combination binds to thyroid hormone response elements in nuclear DNA and is responsible for up-regulating general protein synthesis and inducing a state of positive nitrogen balance. In the embryo, thyroid hormone is necessary for normal development. Hypothyroidism in the embryo is responsible for cretinism, which is characterized by multiple congenital defects and mental retardation.
Thyroid stimulating autoantibodies (TSAb) also activate the human thyroid TSH receptor, leading to the hyperthyroidism of Graves' disease. TSAbs bind to the TSH receptor and mimic the TSH stimulation of the gland by increasing intracellular cAMP.
The feedback loop that regulates T3 and T4 production is a single short negative loop, with the T3 and T4 being responsible for down-regulating pituitary TSH secretion. Meanwhile, continuously secreted hypothalamic TRH is responsible for up-regulating TSH production. The TSH actually secreted by thyrotropes is the net result of the negative effects of T3 and T4 and the positive effect of TRH.
back to the top
The Pro-Opiomelanocortin Family
The POMC gene is expressed in both the anterior and intermediate lobes of the pituitary gland. The primary protein product of the POMC gene is a 285 amino acid precursor that can undergo differential processing to yield at least 8 peptides, dependent upon the location of synthesis and the stimulus leading to their production.
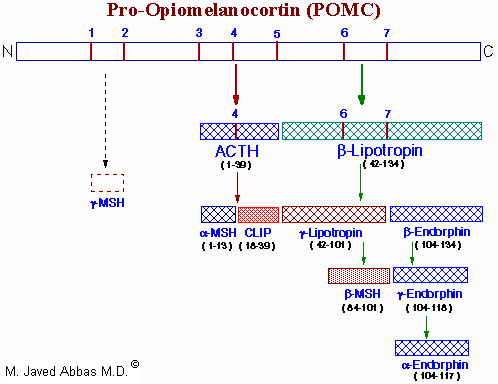 |
| Processing of the POMC precursor protein. Cleavage sites are indicated by the numbers 1 to 7 and consist of the sequences, Arg-Lys, Lys-Arg or Lys-Lys. Adrenocorticotropic hormone (ACTH) and b-lipotropin are products generated in the corticotrophic cells of the anterior pituitary under the control of corticotropin releasing hormone (CRH). Alpha-melanocyte stimulating hormone (a-MSH), corticotropin-like intermediate lobe peptide (CLIP), g-lipotropin and b-endorphin are products generated in the intermediate lobe of the pituitary under the control of dopamine. a-, b- and g-MSH are collectively referred to as melanotropin or intermedin. The numbers in parentheses below each hormone indicate the amino acids of POMC present in each. The N-terminus of ACTH is given as amino acid number 1. The presence and function of g-MSH is unclear hence the dotted lines. Actions, if any, of CLIP and b-lipotropin are also unclear. |
Cortricotropin releasing hormone (CRH) induces rapid secretion of adrenocorticotropic hormone (ACTH, also called corticotropin) and a variety of other peptides from corticotropes of the anterior pituitary. ACTH, a 39 amino acid peptide, is the main physiologically active product of CRH activity. ACTH is derived by post-translational modification from a 241 amino acid precursor known as pro-opiomelanocortin (POMC). The rapid CRH-stimulated secretion of ACTH is associated with induction of adenylate cyclase activity and an increase in cAMP and PKA in corticotropes. Longer-term responses of corticotropes to CRH include a marked increase in POMC mRNA.
The processing of POMC involves glycosylations, acetylations, and extensive proteolytic cleavage at sites shown to contain regions of basic protein sequences. The proteases that recognize these cleavage sites are tissue-specific; thus, the physiologically active product of the anterior pituitary is ACTH. The peptides b-lipotropin (b-LPH) and CLIP and have unknown activity in humans.
Many of the other POMC products are synthesized in other neural tissue that contains proteases with appropriate specificity. In human embryos and in pregnant women, the intermediate lobe is active and leads to the production of endorphins and enkephalins. These same endorphin-producing pathways are active in other neural tissue, and since they bind to the opiate receptors in other parts of the brain they are assumed to represent natural opiate-like analgesic compounds.
The biological role of ACTH is to stimulate the production of adrenal cortex steroids, principally cortisol and costicosterone. The mechanism of action of ACTH involves activation of adenylate cyclase, elevation of cAMP, and increased PKA activity of adrenal cortex tissue. The main effect of these events is to increase the activity of the P450-linked side chain-cleaving enzyme (P450SSC), which converts cholesterol to pregnenolone during steroid hormone synthesis. Because of the distribution of enzymes in the various adrenal cortex subdivisions, the principal physiological effect of ACTH is production of the glucocorticosteroids.
back to the top
Vasopressin and Oxytocin
The principal hormones of the posterior pituitary are the nonapeptides oxytocin and vasopressin. These substances are synthesized as prohormones in neural cell bodies of the hypothalamus and mature as they pass down axons in association with carrier proteins termed neurophysins. The axons terminate in the posterior pituitary, and the hormones are secreted directly into the systemic circulation. Vasopressin is also known as antidiuretic hormone (ADH), because it is the main regulator of body fluid osmolarity. The secretion of vasopressin is regulated in the hypothalamus by osmoreceptors, which sense water concentration and stimulate increased vasopressin secretion when plasma osmolarity increases. The secreted vasopressin increases the reabsorption rate of water in kidney tubule cells, causing the excretion of urine that is concentrated in Na+ and thus yielding a net drop in osmolarity of body fluids. Vasopressin deficiency leads to watery urine and polydipsia, a condition known as diabetes insipidus. Vasopressin binds plasma membrane receptors and acts through G-proteins to activate the cAMP/PKA regulatory system.
The mechanism of action of oxytocin is unknown. Oxytocin secretion in nursing women is stimulated by direct neural feedback obtained by stimulation of the nipple during suckling. Its physiological effects include the contraction of mammary gland myoepithelial cells, which induces the ejection of milk from mammary glands, and the stimulation of uterine smooth muscle contraction leading to childbirth. The remaining hormones reviewed in this chapter are not coupled to tropic neuropeptides, but each has its own regulatory feedback loop that involves the hormone, a plasma component, and a target tissue.
back to the top
Natriuretic Hormones
Natriuresis refers to enhanced urinary excretion of sodium. This can occur in certain disease states and through the action of diuretic drugs. At least 3 natriuretic hormones have been identified. Atrial natiuretic peptide (ANP) was the first cardiac natriuretic hormone identified. This hormone is secreted by cardiac muscle when sodium chloride intake is increased and when the volume of the extracellular fluid expands. Active ANP is a 28-amino acid peptide containing a 17-amino acid ring formed by intrachain disulfide bonding. Two smaller forms of ANP have also been isolated from the brain. A brain natriuretic peptide (BNP) (first isolated from porcine brain) has been identified and found in human heart and blood (but not human brain). BNP has different amino acids in its 17-amino acid ring and is encode for by a different gene. In humans, a third natriuretic peptide (CNP) is present in the brain but not in the heart.
The action of ANP is to cause natriuesis presumably by increasing glomerular filtration rate (its exact mechanism of action remains unclear). ANP induces relaxation of the mesangial cells of the glomeruli and thus may increase the surface area of these cells so that filtration is increased. Alternatively, ANP might act on tubule cells to increase sodium excretion. Other effects of ANP include reducing blood pressure, decreasing the responsiveness of adrenal glomerulosa cells to stimuli that result in aldostreone production and secretion, inhibit secretion of vasopressin and decreasing vascular smooth muscle cell responses to vasoconstrictive agents. These latter actions of ANP are counter to the effects of angiotensin II. In fact, ANP also lowers renin secretion by the kidneys thus lowering circulating angiotensin II levels.
Three different ANP receptors have been identified: ANPR-A, ANPR-B and ANPR-C. When ANP, BNP or CNP bind to receptor, an increase in guanylate cyclase activity results leading to production of cyclic GMP (cGMP). Both ANPR-A and ANPR-B proteins span the plasma membrane and their intracellular domains possess intrinsic guanylate cyclase activity. The exact function of the ANPR-C protein is unclear as this receptor does not contian an intracellular domain with intrinsic guanylylate cyclase activity. It is hypothesized that it may act through a G-protein that activates PLC-g and inhibits adenylate cyclase or that it acts simply as a clearance receptor removing natriuretic peptides from the blood.
back to the top
Renin-Angiotensin System
The renin-angiotensin system is responsible for regulation of blood pressure. A rise in pressure results in the release of renin from the juxtaglomerular cells of the kidneys. The only function for renin is to cleave a 10-amino acid peptide from the N-terminal end of angiotensinogen. This decapeptide is called angiotensin I. Angiotensin I is then cleaved by the action of angiotensin-converting enzyme, ACE to the active hormone, angiotensin II. ACE removes 2 amino acids from the C-terminal end of angiotensin I.
Angiotensin II was also refered to as hypertensin and angiotonin. It is one of the most potent naturally occuring vasoconstrictors. The vasoconstrictive action of angiotensin II is primarily exerted on the arterioles and leads to a rise in both systolic and diastolic blood pressure. In individuals that are depleted of sodium or who have liver disease (e.g. cirrhosis), the pressive actions of angiotensin II are greatly reduced. These conditions lead to increased circulating levels of angiotensin II which in turn leads to a down-regulation in the numbers of angiotensin II receptors on smooth muscle cells. As a consequence, administration of exogenous angiotensin II to these individuals has little effect.
Other physiological responses to angiotensin II include induction of adrenal cortex synthesis and secretion of aldosterone. Angiotensin II also acts on the brain leading to increased blood pressure, vasopressin and ACTH secretion and increased water intake. Angiotensin II affects the contractility of the mesangial cells of the kidney leading to decreased glomerular filtration rate. One additional effect of angiotensin II is to potentiate the release of norepinephrine.
Two distinct types of angiotensin II receptors have been identified, AT1 and AT2. The AT1 receptors are classical serpentine (7 transmembrane spanning) receptors. The AT1 receptors are coupled to a G-protein that leads to activation of PLC-g. Although the AT2 receptors are also serpentine, they do not appear to be coupled to activation of G-proteins.
back to the top
Parathyroid Hormone (PTH)
Parathyroid hormone (PTH, molecular weight 9,500) is synthesized and secreted by chief cells of the parathyroid in response to systemic Ca2+ levels. The Ca2+ receptor of the parathyroid gland responds to Ca2+ by increasing intracellular levels of PKC, Ca2+ and IP3; this stage is followed, after a period of protein synthesis, by PTH secretion. The synthesis and secretion of PTH in chief cells is constitutive, but Ca2+ regulates the level of PTH in chief cells (and thus its secretion) by increasing the rate of PTH proteolysis when plasma Ca2+ levels rise and by decreasing the proteolysis of PTH when Ca2+ levels fall. The role of PTH is to regulate Ca2+ concentration in extracellular fluids. The feedback loop that regulates PTH secretion therefore involves the parathyroids, Ca2+, and the target tissues described below.
PTH acts by binding to cAMP-coupled plasma membrane receptors, initiating a cascade of reactions that culminates in the biological response. The body response to PTH is complex but is aimed in all tissues at increasing Ca2+ levels in extracellular fluids. PTH induces the dissolution of bone by stimulating osteoclast activity, which leads to elevated plasma Ca2+ and phosphate. In the kidney, PTH reduces renal Ca2+ clearance by stimulating its reabsorption; at the same time, PTH reduces the reabsorption of phosphate and thereby increases its clearance. Finally, PTH acts on the liver, kidney, and intestine to stimulate the production of the steroid hormone 1,25-dihydroxycholecalciferol (calcitriol), which is responsible for Ca2+ absorption in the intestine.
back to the top
Calcitonin (CT)
Calcitonin (CT) is a 32-amino acid peptide secreted by C cells of the thyroid gland. Calcitonin is employed therapeutically to relieve the symptoms of osteoporosis, although details of its mechanism of action remain unclear. However, it has been observed that CT induces the synthesis of PTH in isolated cells, which leads in vivo to increased plasma Ca2+ levels. In addition, CT has been shown to reduce the synthesis of osteoporin (Opn), a protein made by osteoclasts and responsible for attaching osteoclasts to bone. Thus, is appears that CT elevates plasma Ca2+ via PTH induction and reduces bone reabsorption by decreasing osteoclast binding to bone.
back to the top
Insulin, Glucagon and Somatostatin
The principal role of the pancreatic hormones is the regulation of whole-body energy metabolism, principally by regulating the concentration and activity of numerous enzymes involved in catabolism and anabolism of the major cell energy supplies.
The earliest of these hormones recognized was insulin, whose major function is to counter the concerted action of a number of hyperglycemia-generating hormones and to maintain low blood glucose levels. Because there are numerous hyperglycemic hormones, untreated disorders associated with insulin generally lead to severe hyperglycemia and shortened life span. Insulin is a member of a family of structurally and functionally similar molecules that include the insulin-like growth factors (IGF-1 and IGF-2), and relaxin. The tertiary structure of all 4 molecules is similar, and all have growth-promoting activities, but the dominant role of insulin is metabolic while the dominant roles of the IGFs and relaxin are in the regulation of cell growth and differentiation.
Insulin is synthesized as a preprohormone in the b cells of the islets of Langerhans. Its signal peptide is removed in the cisternae of the endoplasmic reticulum and it is packaged into secretory vesicles in the Golgi, folded to its native structure, and locked in this conformation by the formation of 2 disulfide bonds. Specific protease activity cleaves the center third of the molecule, which dissociates as C peptide, leaving the amino terminal B peptide disulfide bonded to the carboxy terminal A peptide.
Insulin secretion from b cells is principally regulated by plasma glucose levels, but the precise mechanism by which the glucose signal is transduced remains unclear. One possibility is that the increased uptake of glucose by pancreatic b-cells leads to a concommitant increase in metabolism. The increase in metabolism leads to an elevation in the ATP/ADP ratio. This in turn leads to an inhibition of an ATP-sensitive K+ channel. The net result is a depolarization of the cell leading to Ca2+ influx and insulin secretion.
Chronic increases in numerous other hormones (including GH, hPL, estrogens, and progestins), up-regulate insulin secretion, probably by increasing the preproinsulin mRNA and enzymes involved in processing the increased preprohormone. In contrast, epinephrine diminishes insulin secretion by a cAMP-coupled regulatory path. In addition, epinephrine counters the effect of insulin in liver and peripheral tissue, where it binds to b-adrenergic receptors, induces adenylate cycles activity, increases cAMP, and activates PKA. The latter events induce glycogenolysis and gluconeogenesis, both of which are hyperglycemic and which thus counter insulin's effect on blood glucose levels.
Insulin secreted by the pancreas is directly infused via the portal vein to the liver, where it exerts profound metabolic effects. In most other tissues insulin increases the number of plasma membrane glucose transporters, but in liver glucose uptake is dramatically increased because of increased activity of the enzymes glucokinase, phosphofructokinase-1 (PFK-1), and pyruvate kinase (PK), the key regulatory enzymes of glycolysis. The latter effects are induced by insulin-dependent activation of phosphodiesterase, with decreased PKA activity and diminished phosphorylation of the regulatory glycolytic enzymes. In addition, phophatases specific for the phosphorylated forms of the glycolytic enzymes increase in activity under the influence of insulin. All these events lead to conversion of the glycolytic enzymes to their active forms and consequently a significant increase in glycolysis. In addition, glucose-6-phosphatase activity is down-regulated. The net effect is an increase in the content of hepatocyte glucose and its phosphorylated derivatives, with diminished blood glucose.
In addition to the latter events, diminished cAMP and elevated phosphatase activity combine to convert glycogen phosphorylase to its inactive form and glycogen synthase to its active form, with the result that not only is glucose funneled to glycolytic products, but glycogen content is increased as well.
Insulin generates its intracellular effects by binding to a plasma membrane receptor, which is the same in all cells. The receptor is a disulfide-bonded glycoprotein. One function of insulin (aside from its role in signal transduction) is to increase glucose transport in extrahepatic tissue is by increasing the number of glucose transport molecules in the plasma membrane. Glucose transporters are in a continuous state of turnover. Increases in the plasma membrane content of transporters stem from an increase in the rate of recruitment of new transporters into the plasma membrane, deriving from a special pool of preformed transporters localized in the cytoplasm.
In addition to its role in regulating glucose metabolism, insulin stimulates lipogenesis, diminishes lipolysis, and increases amino acid transport into cells. Insulin also modulates transcription, altering the cell content of numerous mRNAs. It stimulates growth, DNA synthesis, and cell replication, effects that it holds in common with the IGFs and relaxin.
Glucagon is a 29--amino acid hormone synthesized by the a cells of the islets of Langerhans as a very much larger proglucagon molecule. Like insulin, glucagon lacks a plasma carrier protein, and like insulin its circulating half life is also about 5 minutes. As a consequence of the latter trait, the principal effect of glucagon is on the liver, which is the first tissue perfused by blood containing pancreatic secretions. The role of glucagon is well established. It binds to plasma membrane receptors and is coupled through G-proteins to adenylate cyclase. The resultant increases in cAMP and PKA reverse all of the effects described above that insulin has on liver. The increases also lead to a marked elevation of circulating glucose, with the glucose being derived from liver gluconeogenesis and liver glycogenolysis.
Somatostatin, secreted by d cells of the pancreas, is a 14--amino acid peptide, identical to somatostatin secreted by the hypothalamus. In neural tissue somatostatin inhibits GH secretion and thus has systemic effects. In the pancreas, somatostatin acts a paracrine inhibitor of other pancreatic hormones and thus also has systemic effects. It has been speculated that somatostatin secretion responds principally to blood glucose levels, increasing as blood glucose levels rise and thus leading to down-regulation of glucagon secretion.
back to the top
Gastrointestinal Hormones
| Hormone |
Location |
Major Action |
| Gastrin |
gastric antrum, duodenum |
gastric acid and pepsin secretion |
| Cholecystokinin (CCK) |
duodenum, jejunum |
pancreatic amylase secretion |
| | Secretin |
duodenum, jejunum |
pancreatic bicarbonate secretion |
| Gastric inhibitory peptide (GIP) |
small bowel |
enhances glucose-mediated insulin relaese; inhibits gastric acid secretion |
| Vasoactive intestinal peptide (VIP) |
pancreas |
smooth muscle relaxation; stimulates pancreatic bicarbonate secretion |
| Motilin |
small bowel |
initiates interdigestive intestinal motility |
| Pancreatic polypeptide (PP) |
pancreas |
inhibits pancreatic bicarbonate and protein secretion |
| Enkephalins |
stomach, duodenum, gallbladder |
opiate-like actions |
| Substance P |
entire gastrointestinal tract |
physiological actions uncertain |
| Bombesin-like immunoreactivity (BLI) |
stomach, duodenum |
stimulates release of gastrin and CCK |
| Neurotensin |
ileum |
physiological actions unknown |
| Enteroglucagon |
pancreas, small intestine |
physiological actions unknown |
back to the top
Back to Topics<<<<
This article has been modified by Dr. M. Javed Abbas.
If you have any comments please do not hesitate to sign my Guest Book.
20:51 21/12/2002
|
