Introduction
With the exception of retinoic acid, the steroid hormones are all derived from cholesterol. Moreover, with the exception of vitamin D, they all contain the same cyclopentanophenanthrene ring and atomic numbering system as cholesterol. The conversion of C27 cholesterol to the 18-, 19-, and 21-carbon steroid hormones (designated by the nomenclature C with a subscript number indicating the number of carbon atoms, e.g. C19 for androstanes) involves the rate-limiting, irreversible cleavage of a 6-carbon residue from cholesterol, producing pregnenolone (C21) plus isocaproaldehyde.
Common names of the steroid hormones are widely recognized, but systematic nomenclature is gaining acceptance and familiarity with both nomenclatures is increasingly important. Steroids with 21 carbon atoms are known systematically as pregnanes, whereas those containing 19 and 18 carbon atoms are known as androstanes and estranes, respectively. The important mammalian steroid hormones are shown below along with the structure of the precursor, pregneolone. Retinoic acid and vitamin D are not derived from pregnenolone, but from vitamin A and cholesterol respectively.
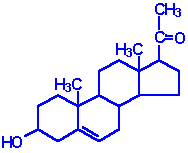 | Pregnenolone: produced directly from cholesterol, the precusor molecule for all C18, C19 and C21 steroids |
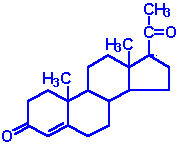 | Progesterone: a progestin, produced directly from pregnenolone and secreted from the corpus luteum, responsible for changes associated with luteral phase of the menstral cycle, differentiation factor for mammary glands |
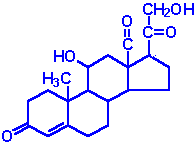 | Aldosterone: the principal mineralocorticoid, produced from progesterone in the zona glomerulosa of adrenal cortex, raises blood pressure and fluid volume, increases Na+ uptake |
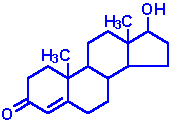 | Testosterone: an androgen, male sex hormone synthesized in the testes, responsible for secondary male sex characteristics, produced from progesterone |
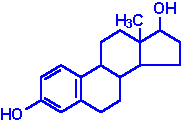 | Estradiol: an estrogen, principal female sex hormone, produced in the ovary, responsible for secondary female sex characteristics |
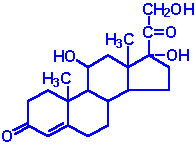 | Cortisol: dominant glucocorticoid in humans, synthesized from progesterone in the zona fasciculata of the adrenal cortex, involved in stress adaptation, elevates blood pressure and Na+ uptake, numerous effects on the immune system |
All the steroid hormones exert their action by passing through the plasma membrane and binding to intracellular receptors. The mechanism of action of the thyroid hormones is similar; they interact with intracellular receptors. Both the steroid and thyroid hormone-receptor complexes exert their action by binding to specific nucleotide squences in the DNA of responsive genes. These DNA sequences are identified as hormone response elements, HREs. The interaction of steroid-receptor complexes with DNA leads to altered rates of transcription of the associated genes.
back to the top
Steroid Hormone Biosynthesis Reactions
The particular steroid hormone class synthesized by a given cell type depends upon its complement of peptide hormone receptors, its response to peptide hormone stimulation and its genetically expressed complement of enzymes. The following indicates which peptide hormone is responsible for stimulating the synthesis of which steroid hormone:
- Luteinizing Hormone (LH):
- progesterone and testosterone
- Adrenocorticotropic hormone (ACTH):
- Follicle Stimulating Hormone (FSH):
- Angiotensin II/III:
The first reaction in converting cholesterol to C18, C19 and C21 steroids involves the cleavage of a 6-carbon group from cholesterol and is the principal committing, regulated, and rate-limiting step in steroid biosynthesis. The enzyme system that catalyzes the cleavage reaction is known as P450-linked side chain cleaving enzyme (P450ssc), or desmolase, and is found in the mitochondria of steroid-producing cells, but not in significant quantities in other cells.
Mitochondrial desmolase is a complex enzyme system consisting of cytochrome P450, and adrenadoxin (a P450 reductant). The activity of each of these components is increased by 2 principal cAMP- and PKA-dependent processes. First, cAMP stimulates PKA, leading to the phosphorylation of a cholesteryl-ester esterase and generating increased concentrations of cholesterol, the substrate for desmolase. Second, long-term regulation is effected at the level the gene for desmolase. This gene contains a cAMP regulatory element (CRE) that binds cAMP and increases the level of desmolase RNA transcription, thereby leading to increased levels of the enzyme. Finally, cholesterol is a negative feedback regulator of HMG CoA reductase activity (see regulation of cholesterol synthesis). Thus, when cytosolic cholesterol is depleted, de novo cholesterol synthesis is stimulated by freeing HMG CoA reductase of its feedback constraints. Subsequent to desmolase activity, pregnenolone moves to the cytosol, where further processing depends on the cell (tissue) under consideration.
The various hydroxylases involved in the synthesis of the steroid hormones have a nomenclature that indicates the site of hydroxylation (e.g. 17a-hydroxylase introduces a hydroxyl group to carbon 17). These hydroxylase enzymes are members of the cytochrome P450 class of enzymes and as such also have a nomenclature indicative of the site of hydroxylation in addition to being identified as P450 class enzymes (e.g. the 17a-hydroxylase is also identified as P450c17).
back to the top
Steroids of the Adrenal Cortex
The adrenal cortex is responsible for production of 3 major classes of steroid hormones: glucocorticoids, which regulate carbohydrate metabolism; mineralocorticoids, which regulate the body levels of sodium and potassium; and androgens, whose actions are similar to that of steroids produced by the male gonads. Adrenal insufficiency is known as Addison disease, and in the absence of steroid hormone replacement therapy can rapidly cause death (in 1--2 weeks).
The adrenal cortex is composed of 3 main tissue regions: zona glomerulosa, zona fasciculata, and zona reticularis. Although the pathway to pregnenolone synthesis is the same in all zones of the cortex, the zones are histologically and enzymatically distinct, with the exact steroid hormone product dependent on the enzymes present in the cells of each zone.
 |
| Synthesis of the various adrenal steroid hormones from cholesterol. Only the terminal hormone structures are included. 3b-DH is 3b-dehydrogenase, P450c11 is 11b-hydroxylase, P450c17 is 17a-hydroxylase, P450c21 is 21b-hydroxylase. |
Zona glomerulosa cells lack the P450c17 that converts pregnenolone and progesterone to their C17 hydroxylated analogs. Thus, the pathways to the glucocorticoids (deoxycortisol and cortisol) and the androgens [dehydroepiandosterone (DHEA) and androstenedione] are blocked in these cells. Zona glomerulosa cells are unique in the adrenal cortex in containing the enzyme responsible for converting corticosterone to aldosterone, the principal and most potent mineralocorticoid. This enzyme is P450c18 (or 18a-hydroxylase), also called aldosterone synthase. The result is that the zona glomerulosa is mainly responsible for the conversion of cholesterol to the weak mineralocorticoid, corticosterone and the principal mineralocorticoid, aldosterone.
Cells of the zona fasciculata and zona reticularis lack aldosterone synthase (P450c18) that converts corticosterone to aldosterone, and thus these tissues produce only the weak mineralocorticoid corticosterone. However, both these zones do contain the P450c17 missing in zona glomerulosa and thus produce the major glucocorticoid, cortisol. Zona fasciculata and zona reticularis cells also contain the C17,20 lyase, whose activity is responsible for producing the androgens, dehydroepiandosterone (DHEA) and androstenedione. Thus, fasciculata and reticularis cells can make corticosteroids and the adrenal androgens, but not aldosterone.
As noted earlier, P450ssc is a mitochondrial activity. Its product, pregnenolone, moves to the cytosol, where it is converted either to androgens or to 11-deoxycortisol and 11-deoxycorticosterone by enzymes of the endoplasmic reticulum. The latter 2 compounds then re-enter the mitochondrion, where the enzymes are located for tissue-specific conversion to glucocorticoids or mineralocorticoids, respectively.
back to the top
Regulation of Adrenal Steroid Synthesis
Adrenocorticotropic hormone (ACTH) of the hypothalamus regulates the hormone production of the zona fasciculata and zona reticularis. ACTH receptors in the plasma membrane activate adenylate cyclase with production of the second messenger, cAMP. The effect of ACTH on the production of cortisol is particularly important, with the result that a classic feedback loop is prominent in regulating the circulating levels of corticotropin releasing hormone, (CRH), ACTH, and cortisol.
Mineralocorticoid secretion from the zona glomerulosa is stimulated by an entirely different mechanism. Angiotensins II and III, derived from the action of
the kidney protease renin on liver-derived angiotensinogen, stimulate zona glomerulosa cells by binding a plasma membrane receptor coupled to phospholipase C. Thus, angiotensin II and III binding to their receptor leads to the activation of PKC and elevated intracellular Ca2+ levels. These events lead to increased P450ssc activity and increased production of aldosterone. In the kidney, aldosterone regulates sodium retention by stimulating gene expression of mRNA for the Na+/K+-ATPase responsible for the reaccumulation of sodium from the urine.
The interplay between renin from the kidney and plasma angiotensinogen is important in regulating plasma aldosterone levels, sodium and potassium levels, and ultimately blood pressure. Among the drugs most widely employed used to lower blood pressure are the angiotensin converting enzyme (ACE) inhibitors. These compounds are potent competitive inhibitors of the enzyme that converts angiotensin I to the physiologically active angiotensins II and III. This feedback loop is closed by potassium, which is a potent stimulator of aldosterone secretion. Changes in plasma potassium of as little as 0.1 millimolar can cause wide fluctuations (+/- 50%) in plasma levels of aldosterone. Potassium increases aldosterone secretion by depolarizing the plasma membrane of zona glomerulosa cells and opening a voltage-gated calcium channel, with a resultant increase in cytoplasmic calcium and the stimulation of calcium-dependent processes.
Although fasciculata and reticularis cells each have the capability of synthesizing androgens and glucocorticoids, the main pathway normally followed is that leading to glucocorticoid production. However, when genetic defects occur in the 3 enzyme complexes leading to glucocorticoid production, large amounts of the most important androgen, dehydroepiandrosterone (DHEA), are produced. These lead to hirsutism and other masculinizing changes in secondary sex characteristics.
back to the top
Gonadal Steroid Hormones
Although many steroids are produced by the testes and the ovaries, the two most important are testosterone and estradiol. These compounds are under tight biosynthetic control, with short and long negative feedback loops that regulate the secretion of follicle stimulating hormone (FSH) and leutinizing hormone (LH) by the pituitary and gonadotropin releasing hormone (GnRH) by the hypothalamus. Low levels of circulating sex hormone reduce feedback inhibition on GnRH synthesis (the long loop), leading to elevated FSH and LH. The latter peptide hormones bind to gonadal tissue and stimulate P450ssc activity, resulting in sex hormone production via cAMP and PKA mediated pathways. The roles of cAMP and PKA in gonadal tissue are the same as that described for glucocorticoid production in the adrenals, but in this case adenylate cyclase activation is coupled to the binding of LH to plasma membrane receptors.
The biosynthetic pathway to sex hormones in male and female gonadal tissue includes the production of the androgens---androstenedione and dehydroepiandrosterone. Testes and ovaries contain an additional enzyme, a 17b-hydroxysteroid dehydrogenase, that enables androgens to be converted to testosterone
In males, LH binds to Leydig cells, stimulating production of the principal Leydig cell hormone, testosterone. Testosterone is secreted to the plasma and also carried to Sertoli cells by androgen binding protein (ABP). In Sertoli cells the D-4 double bond of testosterone is reduced, producing dihydrotestosterone. Testosterone and dihydrotestosterone are carried in the plasma, and delivered to target tissue, by a specific gonadal-steroid binding globulin (GBG). In a number of target tissues, testosterone can be converted to dihydrotestosterone (DHT). DHT is the most potent of the male steroid hormones, with an activity that is 10 times that of testosterone. Because of its relatively lower potency, testosterone is sometimes considered to be a prohormone.
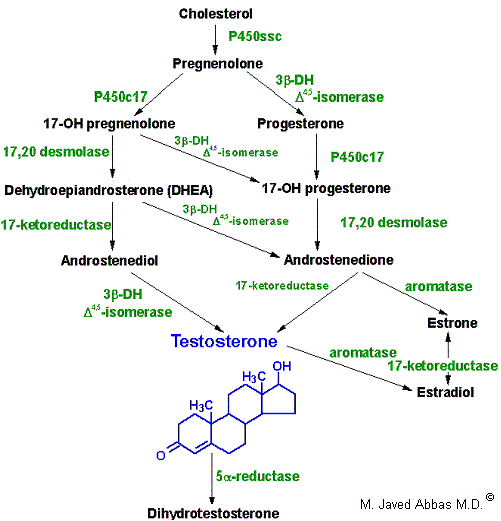 |
| Synthesis of the male sex hormones in Leydig cells of the testis. P450SSC , 3b-DH, and P450c17 are the same enzymes as those needed for adrenal steroid hormone synthesis. 17,20-desmolase is the same as 17,20-lyase of adrenal hormone synthesis.. |
Testosterone is also produced by Sertoli cells but in these cells it is regulated by FSH, again acting through a cAMP- and PKA-regulatory pathway. In addition, FSH stimulates Sertoli cells to secrete androgen-binding protein (ABP), which transports testosterone and DHT from Leydig cells to sites of spermatogenesis. There, testosterone acts to stimulate protein synthesis and sperm development.
In females, LH binds to thecal cells of the ovary, where it stimulates the synthesis of androstenedione and testosterone by the usual cAMP- and PKA-regulated pathway. An additional enzyme complex known as aromatase is responsible for the final conversion of the latter 2 molecules into the estrogens. Aromatase is a complex endoplasmic reticulum enzyme found in the ovary and in numerous other tissues in both males and females. Its action involves hydroxylations and dehydrations that culminate in aromatization of the A ring of the androgens.
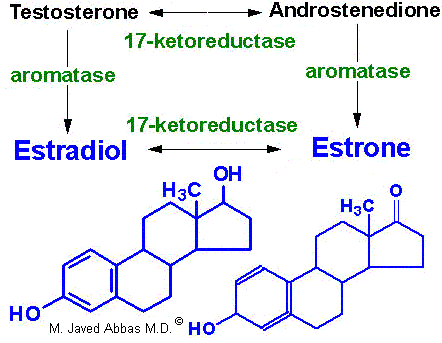 |
| Synthesis of the major female sex hormones in the ovary. Synthesis of testosterone and androstenedione from cholesterol occurs by the same pathways as indicated for synthesis of the male sex hormones. |
Aromatase activity is also found in granulosa cells, but in these cells the activity is stimulated by FSH. Normally, thecal cell androgens produced in response to LH diffuse to granulosa cells, where granulosa cell aromatase converts these androgens to estrogens. As granulosa cells mature they develop competent large numbers of LH receptors in the plasma membrane and become increasingly responsive to LH, increasing the quantity of estrogen produced from these cells. Granulosa cell estrogens are largely, if not all, secreted into follicular fluid. Thecal cell estrogens are secreted largely into the circulation, where they are delivered to target tissue by the same globulin (GBG) used to transport testosterone.
back to the top
Steroid Hormone Receptors
The receptors to which steroid hormones bind are ligand-activated proteins that regulate transcription of selected genes. Unlike
peptide hormone receptors, that span the plasma membrane and bind ligand outside the cell, steroid hormone
receptors are found in the cytosol and the nucleus. The steroid hormone receptors belong to the steroid and thyroid hormone receptor super-family of
proteins, that includes receptors for steroid hormones, thyroid hormones, vitamin D and vitamin A (retinoic acid).
When these receptors bind ligand they undergo a conformational change that renders them activated to recognize and bind to specific nucleotide sequences. These specific nucleotide sequences in the DNA
are referred to as hormone-response elements (HREs). When ligand-receptor complexes interact with DNA they alter the
transcriptional level (responses can be either activating or repressing) of the associated gene. Thus, the steroid-thyroid family of receptors all have three distinct
domains: a ligand-binding domain, a DNA-binding domain and a transcriptional regulatory domain. Although there is the commonly observed effect of altered
transcriptional activity in response to hormone-receptor interaction, there are family member-specific effects with ligand-receptor interaction. Binding of
thyroid hormone to its receptor results in release of the receptor from DNA. Several receptors are induced to interact with other transcriptional mediators
in response to ligand binding. Binding of glucocorticoid leads to translocation of the ligand-receptor complex from the cytosol to the nucleus.
The receptors for the retinoids (vitamin A and its derivatives) are identified as RARs (for retinoic acid, RA receptors)
and exist in at least three subtypes, RARa, RARb and RARg. In addition, there is another
family of nuclear receptors termed the retinoid X receptors (RXRs) that represents a second class of retinoid-responsive transcription factors. The RXRs have been shown to enhance the DNA-binding activity of RARs and the
thyroid hormone receptors (TRs). There are also three distinct RXRs (a, b and g). The major difference between the
RARs and RXRs is that the former exhibit highest affinity for all-trans-retinoic acid (all-trans-RA) and the latter for 9-cis-RA.
Additional super-family members are the peroxisome proliferator-activated receptors (PPARs). These receptors were originally discovered as
proteins activated by agents that stimulate proliferation of peroxisomes in rat liver. An intracellular lipid-binding protein identified as aP2 is expressed
exclusively in differentiated adipocytes. An adipocyte-specific enhancer of the aP2 gene is a target for peroxisome proliferators, fatty acids and 9-cis-RA. Subsequent to these observations
it was found that there is an adipocyte-specific PPAR family identified as PPARg. The PPARg proteins form heterodimers with
RXRs to activate adipocyte-specific enhancers such as the one in the aP2 gene.
Recent evidence has demonstrated a role for PPARg proteins in the etiology of type 2 diabetes.
A relatively new class of drugs used to increase the sensitivity of the body to insulin are the thiazolidinedione drugs. These compounds bind to and alter the
function of PPARg. Mutations in the gene for PPARg have been correlated with insulin resistance. It is still not completely clear
how impaired PPARg signaling can affect the sensitivity of the body to insulin or indeed if the observed mutations are a direct or indirect
cause of the symptoms of insulin resistance.
back to the top
Back to Topics<<<<
This article has been modified by Dr. M. Javed Abbas.
If you have any comments please do not hesitate to sign my Guest Book.
20:50 21/12/2002
|








